rKLO8, a novel Leishmania donovani - derived recombinant immunodominant protein for sensitive detection of visceral leishmaniasis in Sudan
- PMID: 23875052
- PMCID: PMC3715527
- DOI: 10.1371/journal.pntd.0002322
rKLO8, a novel Leishmania donovani - derived recombinant immunodominant protein for sensitive detection of visceral leishmaniasis in Sudan
Abstract
Background: For effective control of visceral leishmaniasis (VL) in East Africa, new rapid diagnostic tests are required to replace current tests with low sensitivity. The aim of this study is to improve diagnosis of VL in East Africa by testing a new antigen from an autochthonous L. donovani strain in Sudan.
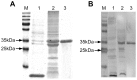
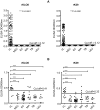
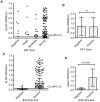
Methodology and principle findings: We cloned, expressed and purified a novel recombinant protein antigen of L. donovani from Sudan, designated rKLO8, that contains putative conserved domains with significant similarity to the immunodominant kinesin proteins of Leishmania. rKLO8 exhibited 93% and 88% amino acid identity with cloned kinesin proteins of L. infantum (synonymous L. chagasi) (K39) and L. donovani (KE16), respectively. We evaluated the diagnostic efficiency of the recombinant protein in ELISA for specific detection of VL patients from Sudan. Data were compared with a rK39 ELISA and two commercial kits, the rK39 strip test and the direct agglutination test (DAT). Of 106 parasitologically confirmed VL sera, 104 (98.1%) were tested positive by rKLO8 as compared to 102 (96.2%) by rK39. Importantly, the patients' sera showed increased reactivity with rKLO8 than rK39. Specificity was 96.1% and 94.8% for rKLO8- and rK39 ELISAs, respectively. DAT showed 100% specificity and 94.3% sensitivity while rK39 strip test performed with 81.1% sensitivity and 98.7% specificity.
Conclusion: The increased reactivity of Sudanese VL sera with the rKLO8 makes this antigen a potential candidate for diagnosis of visceral leishmaniasis in Sudan. However, the suitability at the field level will depend on its performance in a rapid test format.
Conflict of interest statement
I have read the journal's policy and have the following conflicts: rKLO8 is currently filed for patent application.
Figures

References
-
- Lainson R, Shaw JJ (1987) Evolution, classification and geographical distribution. In: Peters W, Killick-Kendrick R, editors. The leishmaniases in biology and medicine. London: Academic Press. 1–120.
-
- Jamjoom MB, Ashford RW, Bates PA, Chance ML, Kemp SJ, et al. (2004) Leishmania donovani is the only cause of visceral leishmaniasis in East Africa; previous descriptions of L. infantum and “L. archibaldi” from this region are a consequence of convergent evolution in the isoenzyme data. Parasitology 129: 399–409. - PubMed
-
- Mauricio IL, Stothard JR, Miles MA (2000) The strange case of Leishmania chagasi. Parasitol Today 16: 188–189. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

