A simple set of validation steps identifies and removes false results in a sandwich enzyme-linked immunosorbent assay caused by anti-animal IgG antibodies in plasma from arthritis patients
- PMID: 23875127
- PMCID: PMC3695686
- DOI: 10.1186/2193-1801-2-263
A simple set of validation steps identifies and removes false results in a sandwich enzyme-linked immunosorbent assay caused by anti-animal IgG antibodies in plasma from arthritis patients
Abstract
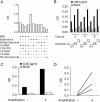
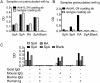
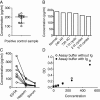
Rheumatoid arthritis (RA) and spondyloarthritis (SpA) are chronic diseases characterized by activation of the immune system and production of antibodies. Thus, rheumatoid factor, anti-animal IgG antibodies and heterophilic antibodies in plasma samples from arthritis patients can interfere with immunoassays such as sandwich enzyme-linked immunosorbent assay (ELISA) systems often used in arthritis research. However, standard methodologies on how to test for false results caused by these antibodies are lacking. The objective of this study was to design a simple set of steps to validate a sandwich ELISA before using it for measuring analytes in plasma from arthritis patients. An interleukin-24 (IL-24) sandwich ELISA system was prepared with a monoclonal mouse capture antibody and a polyclonal goat detection antibody and tested for interference by rheumatoid factor, anti-animal IgG antibodies and heterophilic antibodies. Plasma samples from 23 patients with RA and SpA were used. No differences were found between plasma samples measured in wells coated with anti-IL-24 specific antibody and in wells coated with isotype control antibody (false positive results), and recombinant human IL-24 was not recovered in spiked samples (false negative results). This interference was removed after preincubating the plasma samples from patients with arthritis with goat or bovine IgG, suggesting that anti-animal IgG antibodies found in the plasma of the arthritis patients caused the false results. Additional testing showed that the signal-to-noise ratio could be increased by titration of the capture and detection antibodies and by using the ELAST amplification system. Finally, the calculated concentration of IL-24 was increased in ethylenediaminetetraacetic acid (EDTA) plasma compared to heparin plasma and serum and decreased with repetitive freeze/thaw cycles of the samples illustrating how sample handling could additionally contribute to the variations reported by different laboratories in measurement of the same analyte. This study proposes a simple set of validation steps to evaluate and optimize a sandwich ELISA before using it for measuring analytes in plasma from arthritis patients. Anti-animal IgG antibodies are also present in healthy individuals, suggesting that validation of ELISA systems for measuring non-arthritis samples could also be improved by this simple set of validation steps.
Keywords: Anti-animal IgG antibodies; Arthritis; ELAST amplification system; Enzyme-linked immunosorbent assay; Heterophilic antibodies; Immunoassay; Interference; Multiplex; Rheumatoid factor.
Figures

References
-
- Bartels EM, Ribel-Madsen S. Methods. 2013. Cytokine measurements and possible interference from heterophilic antibodies - Problems and solutions experienced with rheumatoid factor. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

