Interaction of the tick immune system with transmitted pathogens
- PMID: 23875177
- PMCID: PMC3712896
- DOI: 10.3389/fcimb.2013.00026
Interaction of the tick immune system with transmitted pathogens
Abstract
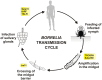
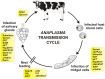
Ticks are hematophagous arachnids transmitting a wide variety of pathogens including viruses, bacteria, and protozoans to their vertebrate hosts. The tick vector competence has to be intimately linked to the ability of transmitted pathogens to evade tick defense mechanisms encountered on their route through the tick body comprising midgut, hemolymph, salivary glands or ovaries. Tick innate immunity is, like in other invertebrates, based on an orchestrated action of humoral and cellular immune responses. The direct antimicrobial defense in ticks is accomplished by a variety of small molecules such as defensins, lysozymes or by tick-specific antimicrobial compounds such as microplusin/hebraein or 5.3-kDa family proteins. Phagocytosis of the invading microbes by tick hemocytes is likely mediated by the primordial complement-like system composed of thioester-containing proteins, fibrinogen-related lectins and convertase-like factors. Moreover, an important role in survival of the ingested microbes seems to be played by host proteins and redox balance maintenance in the tick midgut. Here, we summarize recent knowledge about the major components of tick immune system and focus on their interaction with the relevant tick-transmitted pathogens, represented by spirochetes (Borrelia), rickettsiae (Anaplasma), and protozoans (Babesia). Availability of the tick genomic database and feasibility of functional genomics based on RNA interference greatly contribute to the understanding of molecular and cellular interplay at the tick-pathogen interface and may provide new targets for blocking the transmission of tick pathogens.
Keywords: Anaplasma; Babesia; Borrelia; antimicrobial peptides; innate immunity; phagocytosis; tick; tick-borne diseases.
Figures


References
-
- Antunes S., Galindo R. C., Almazan C., Rudenko N., Golovchenko M., Grubhoffer L., et al. (2012). Functional genomics studies of Rhipicephalus (Boophilus) annulatus ticks in response to infection with the cattle protozoan parasite, Babesia bigemina. Int. J. Parasitol. 42, 187–195 10.1016/j.ijpara.2011.12.003 - DOI - PubMed
-
- Ariki S., Takahara S., Shibata T., Fukuoka T., Ozaki A., Endo Y., et al. (2008). Factor C acts as a lipopolysaccharide-responsive C3 convertase in horseshoe crab complement activation. J. Immunol. 181, 7994–8001 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

