Critical role of calpain in spinal cord degeneration in Parkinson's disease
- PMID: 23875735
- PMCID: PMC3859802
- DOI: 10.1111/jnc.12374
Critical role of calpain in spinal cord degeneration in Parkinson's disease
Abstract
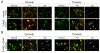
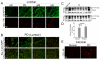
While multiple molecular mechanisms contribute to midbrain nigrostriatal dopaminergic degeneration in Parkinson's disease (PD), the mechanism of damage in non-dopaminergic sites within the central nervous system, including the spinal cord, is not well-understood. Thus, to understand the comprehensive pathophysiology underlying this devastating disease, postmortem spinal cord tissue samples (cervical, thoracic, and lumbar segments) from patients with PD were analyzed compared to age-matched normal subjects or Alzheimer's disease for selective molecular markers of neurodegeneration and inflammation. Distal axonal degeneration, relative abundance of both sensory and motor neuron death, selective loss of ChAT(+) motoneurons, reactive astrogliosis, microgliosis, increased cycloxygenase-2 (Cox-2) expression, and infiltration of T cells were observed in spinal cord of PD patients compared to normal subjects. Biochemical analyses of spinal cord tissues revealed associated inflammatory and proteolytic events (elevated levels of Cox-2, expression and activity of μ- and m-calpain, degradation of axonal neurofilament protein, and concomitantly low levels of endogenous inhibitor - calpastatin) in spinal cord of PD patients. Thus, pathologically upregulated calpain activity in spinal cords of patients with PD may contribute to inflammatory response-mediated neuronal death, leading to motor dysfunction. We proposed calpain over-activation and calpain-calpastatin dysregulation driving in a cascade of inflammatory responses (microglial activation and T cell infiltration) and degenerative pathways culminating in axonal degeneration and neuronal death in spinal cord of Parkinson's disease patients. This may be one of the crucial mechanisms in the degenerative process.
Keywords: axonal degeneration; calpain; inflammation; neurodegeneration.
© 2013 International Society for Neurochemistry.
Figures


References
-
- Banik NL, Hogan EL, Jenkins MG, McDonald JK, McAlhaney WW, Sostek MB. Purification of a calcium-activated neutral proteinase from bovine brain. Neurochem Res. 1983;8:1389–1405. - PubMed
-
- Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. - PubMed
-
- Burguillos MA, Hajji N, Englund E, Persson A, Cenci AM, Machado A, Cano J, Joseph B, Venero JL. Apoptosis-inducing factor mediates dopaminergic cell death in response to LPS-induced inflammatory stimulus: evidence in Parkinson’s disease patients. Neurobiol Dis. 2011;41:177–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

