Synaptodendritic recovery following HIV Tat exposure: neurorestoration by phytoestrogens
- PMID: 23875777
- PMCID: PMC4041987
- DOI: 10.1111/jnc.12375
Synaptodendritic recovery following HIV Tat exposure: neurorestoration by phytoestrogens
Abstract
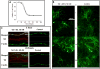
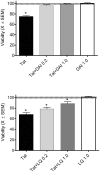
HIV-1 infects the brain and, despite antiretroviral therapy, many infected individuals suffer from HIV-1-associated neurocognitive disorders (HAND). HAND is associated with dendritic simplification and synaptic loss. Prevention of synaptodendritic damage may ameliorate or forestall neurocognitive decline in latent HIV-1 infections. The HIV-1 transactivating protein (Tat) is produced during viral latency in the brain and may cause synaptodendritic damage. This study examined the integrity of the dendritic network after exposure to HIV-1 Tat by labeling filamentous actin (F-actin)-rich structures (puncta) in primary neuronal cultures. After 24 h of treatment, HIV-1 Tat was associated with the dendritic arbor and produced a significant reduction of F-actin-labeled dendritic puncta as well as loss of dendrites. Pre-treatment with either of two plant-derived phytoestrogen compounds (daidzein and liquiritigenin), significantly reduced synaptodendritic damage following HIV-1 Tat treatment. In addition, 6 days after HIV-1 Tat treatment, treatment with either daidzein, or liquiritigenin enhanced recovery, via the estrogen receptor, from HIV-1 Tat-induced synaptodendritic damage. These results suggest that either liquiritigenin or daidzein may not only attenuate acute synaptodendritic injury in HIV-1 but may also promote recovery from synaptodendritic damage. The HIV-1 transactivating protein (Tat) is produced during viral latency in the brain. Treatment with either daidzein or liquiritigenin restored the loss of synaptic connectivity produced by HIV-1 Tat. This neurorestoration was mediated by estrogen receptors (ER). These results suggest that plant-derived phytoestrogens may promote recovery from HIV-1-induced synaptodendritic damage.
Keywords: F-actin; HAND; cell culture; daidzein; liquiritigenin; rat.
© 2013 International Society for Neurochemistry.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




References
-
- Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol. 1999;25:123–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

