SOD therapeutics: latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways
- PMID: 23875805
- PMCID: PMC4005498
- DOI: 10.1089/ars.2012.5147
SOD therapeutics: latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways
Abstract
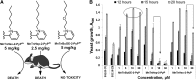
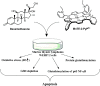
Significance: Superoxide dismutase (SOD) enzymes are indispensable and ubiquitous antioxidant defenses maintaining the steady-state levels of O2·(-); no wonder, thus, that their mimics are remarkably efficacious in essentially any animal model of oxidative stress injuries thus far explored.
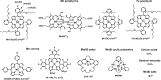
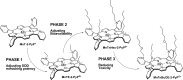
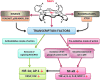
Recent advances: Structure-activity relationship (half-wave reduction potential [E1/2] versus log kcat), originally reported for Mn porphyrins (MnPs), is valid for any other class of SOD mimics, as it is dominated by the superoxide reduction and oxidation potential. The biocompatible E1/2 of ∼+300 mV versus normal hydrogen electrode (NHE) allows powerful SOD mimics as mild oxidants and antioxidants (alike O2·(-)) to readily traffic electrons among reactive species and signaling proteins, serving as fine mediators of redox-based signaling pathways. Based on similar thermodynamics, both SOD enzymes and their mimics undergo similar reactions, however, due to vastly different sterics, with different rate constants.
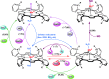
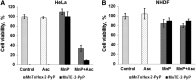
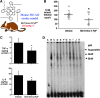
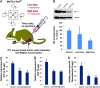
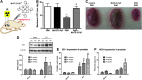
Critical issues: Although log kcat(O2·(-)) is a good measure of therapeutic potential of SOD mimics, discussions of their in vivo mechanisms of actions remain mostly of speculative character. Most recently, the therapeutic and mechanistic relevance of oxidation of ascorbate and glutathionylation and oxidation of protein thiols by MnP-based SOD mimics and subsequent inactivation of nuclear factor κB has been substantiated in rescuing normal and killing cancer cells. Interaction of MnPs with thiols seems to be, at least in part, involved in up-regulation of endogenous antioxidative defenses, leading to the healing of diseased cells.
Future directions: Mechanistic explorations of single and combined therapeutic strategies, along with studies of bioavailability and translational aspects, will comprise future work in optimizing redox-active drugs.
Figures













References
-
- Abashkin YG. and Burt SK. (salen)MnIII compounds as nonpeptidyl mimics of catalase. Mechanism-based tuning of catalase activity: a theoretical study. Inorg Chem 44: 1425–1432, 2005 - PubMed
-
- Abraham SK, Sarma L, and Kesavan PC. Protective effects of chlorogenic acid, curcumin and beta-carotene against gamma-radiation-induced in vivo chromosomal damage. Mutat Res 303: 109–112, 1993 - PubMed
-
- Aitken JB, Shearer EL, Giles NM, Lai B, Vogt S, Reboucas JS, Batinic-Haberle I, Lay PA, and Giles GI. Intracellular targeting and pharmacological activity of the superoxide dismutase mimics MnTE-2-PyP5+ and MnTnHex-2-PyP5+ regulated by their porphyrin ring substituents. Inorg Chem 52: 4121–4123, 2013 - PubMed
-
- Allen BG, Sibenaller ZA, Cullen JJ, Buettner GR, Welsh JJ, Wagner BA, van't Erve TJ, Buatti JM, Carlisle TL, Smith MC, Walsh SA, Bayouth JE, TenNapel M, and Spitz DR. Pharmacological ascorbate enhances chemo-radio-sensitization in brain and lung cancer. Free Radic Biol Med 53: S39, 2012
-
- Andrievsky GV, Bruskov VI, Tykhomyrov AA, and Gudkov SV. Peculiarities of the antioxidant and radioprotective effects of hydrated C60 fullerene nanostuctures in vitro and in vivo. Free Radic Biol Med 47: 786–793, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

