The circadian clock is functional in eosinophils and mast cells
- PMID: 23876110
- PMCID: PMC3839650
- DOI: 10.1111/imm.12157
The circadian clock is functional in eosinophils and mast cells
Abstract
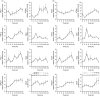
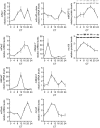
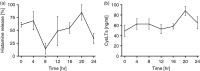
Allergic diseases are frequently exacerbated between midnight and early morning, suggesting a role for the biological clock. Mast cells (MC) and eosinophils are the main effector cells of allergic diseases and some MC-specific or eosinophil-specific markers, such as tryptase or eosinophil cationic protein, exhibit circadian variation. Here, we analysed whether the circadian clock is functional in mouse and human eosinophils and MC. Mouse jejunal MC and polymorphonuclear cells from peripheral blood (PMNC) were isolated around the circadian cycle. Human eosinophils were purified from peripheral blood of non-allergic and allergic subjects. Human MC were purified from intestinal tissue. We found a rhythmic expression of the clock genes mPer1, mPer2, mClock and mBmal1 and eosinophil-specific genes mEcp, mEpo and mMbp in murine PMNC. We also found circadian variations for hPer1, hPer2, hBmal1, hClock, hEdn and hEcp mRNA and eosinophil cationic protein (ECP) in human eosinophils of both healthy and allergic people. Clock genes mPer1, mPer2, mClock and mBmal1 and MC-specific genes mMcpt-5, mMcpt-7, mc-kit and mFcεRI α-chain and protein levels of mMCPT5 and mc-Kit showed robust oscillation in mouse jejunum. Human intestinal MC expressed hPer1, hPer2 and hBmal1 as well as hTryptase and hFcεRI α-chain, in a circadian manner. We found that pre-stored histamine and de novo synthesized cysteinyl leukotrienes, were released in a circadian manner by MC following IgE-mediated activation. In summary, the biological clock controls MC and eosinophils leading to circadian expression and release of their mediators and, hence it might be involved in the pathophysiology of allergy.
Keywords: allergy; biological clock; circadian rhythm; eosinophils; mast cells.
© 2013 John Wiley & Sons Ltd.
Figures


References
-
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41. - PubMed
-
- Froy O, Chapnik N, Miskin R. Mouse intestinal cryptdins exhibit circadian oscillation. FASEB J. 2005;19:1920–2. - PubMed
-
- Froy O, Chapnik N. Circadian oscillation of innate immunity components in mouse small intestine. Mol Immunol. 2007;44:1954–60. - PubMed
-
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

