Knockdown of both mitochondrial isocitrate dehydrogenase enzymes in pancreatic beta cells inhibits insulin secretion
- PMID: 23876293
- PMCID: PMC3923500
- DOI: 10.1016/j.bbagen.2013.07.013
Knockdown of both mitochondrial isocitrate dehydrogenase enzymes in pancreatic beta cells inhibits insulin secretion
Erratum in
- Biochim Biophys Acta. 2013. doi: 10.1016/j.bbagen.2013.09.024. Hasan, Noaman M [added] doi: 10.1016/j.bbagen.2013.09.024
- Biochim Biophys Acta. 2015 Jun;1850(6):1343
Abstract
Background: There are three isocitrate dehydrogenases (IDHs) in the pancreatic insulin cell; IDH1 (cytosolic) and IDH2 (mitochondrial) use NADP(H). IDH3 is mitochondrial, uses NAD(H) and was believed to be the IDH that supports the citric acid cycle.
Methods: With shRNAs targeting mRNAs for these enzymes we generated cell lines from INS-1 832/13 cells with severe (80%-90%) knockdown of the mitochondrial IDHs separately and together in the same cell line.
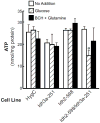
Results: With knockdown of both mitochondrial IDH's mRNA, enzyme activity and protein level, (but not with knockdown of only one mitochondrial IDH) glucose- and BCH (an allosteric activator of glutamate dehydrogenase)-plus-glutamine-stimulated insulin release were inhibited. Cellular levels of citrate, α-ketoglutarate, malate and ATP were altered in patterns consistent with blockage at the mitochondrial IDH reactions. We were able to generate only 50% knockdown of Idh1 mRNA in multiple cell lines (without inhibition of insulin release) possibly because greater knockdown of IDH1 was not compatible with cell line survival.
Conclusions: The mitochondrial IDHs are redundant for insulin secretion. When both enzymes are severely knocked down, their low activities (possibly assisted by transport of IDH products and other metabolic intermediates from the cytosol into mitochondria) are sufficient for cell growth, but inadequate for insulin secretion when the requirement for intermediates is certainly more rapid. The results also indicate that IDH2 can support the citric acid cycle.
General significance: As almost all mammalian cells possess substantial amounts of all three IDH enzymes, the biological principles suggested by these results are probably extrapolatable to many tissues.
Keywords: Cytosolic isocitrate dehydrogenase; INS-1 832/13 cell line; Insulin secretion; Mitochondrial isocitrate dehydrogenase; Stable knockdown of isocitrate dehydrogenase; shRNA.
© 2013.
Figures


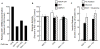




References
-
- MacDonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J Biol Chem. 1995;270:20051–20058. - PubMed
-
- MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab. 2005;288:E1–15. - PubMed
-
- Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000;49:718–726. - PubMed
-
- Flamez D, Berger V, Kruhøffer M, Orntoft T, Pipeleers D, Schuit FC. Critical role for cataplerosis via citrate in glucose-regulated insulin release. Diabetes. 2002;51:2018–2024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

