Surface association of Pht proteins of Streptococcus pneumoniae
- PMID: 23876799
- PMCID: PMC3811752
- DOI: 10.1128/IAI.00562-13
Surface association of Pht proteins of Streptococcus pneumoniae
Abstract
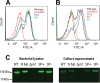
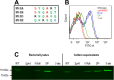
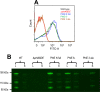
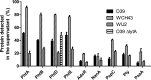
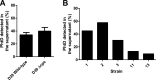
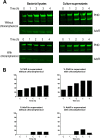
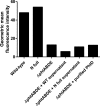
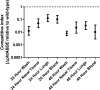
Streptococcus pneumoniae is a major human pathogen responsible for massive global morbidity and mortality. The pneumococcus attaches a variety of proteins to its cell surface, many of which contribute to virulence; one such family are the polyhistidine triad (Pht) proteins PhtA, PhtB, PhtD, and PhtE. In this study, we have examined the mechanism of Pht surface attachment using PhtD as a model. Analysis of deletion and point mutants identified a three-amino-acid region of PhtD (Q27-H28-R29) that is critical for the process. The analogous region in PhtE was also necessary for its attachment to the cell surface. Furthermore, we show that a large proportion of the total amount of each Pht protein is released into bacterial culture supernatants. Other surface proteins were also released, albeit to lesser extents, and this was not due to pneumococcal autolysis. The extent of release of surface proteins was strain dependent and was not affected by the capsule. Lastly, we compared the fitness of wild-type and ΔphtABDE pneumococci in vivo in a mouse coinfection model. Release of Pht proteins by the wild type did not complement the mutant strain, consistent with surface-attached rather than soluble forms of the Pht proteins playing the major role in virulence. The significant degree of release of Pht proteins from intact bacteria may have implications for the use of these proteins in novel vaccines.
Figures
References
-
- Kadioglu A, Weiser JN, Paton JC, Andrew PW. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6:288–301 - PubMed
-
- Pérez-Dorado I, Galan-Bartual S, Hermoso JA. 2012. Pneumococcal surface proteins: when the whole is greater than the sum of its parts. Mol. Oral Microbiol. 27:221–245 - PubMed
-
- Paterson GK, Mitchell TJ. 2004. The biology of Gram-positive sortase enzymes. Trends Microbiol. 12:89–95 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

