Plasma endothelial protein C receptor influences innate immune response in ovarian cancer by decreasing the population of natural killer and TH17 helper cells
- PMID: 23877403
- PMCID: PMC3829768
- DOI: 10.3892/ijo.2013.2021
Plasma endothelial protein C receptor influences innate immune response in ovarian cancer by decreasing the population of natural killer and TH17 helper cells
Abstract
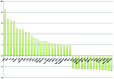
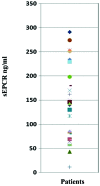
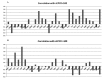
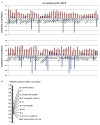
In spite of the growing importance of endothelial protein C receptor/active protein C (EPCR/aPC) in tumor biology, their impact on immunological homeostasis remains largely unexplored. The objective of this study was to assess whether soluble plasma endothelial protein C receptor (sEPCR), which is a regulator of circulating aPC, is involved in innate immune response in cancer patients. In the Ovcar-3 ovarian cancer line, the role of aPC in secretion of cytokines was analyzed. In parallel, in 33 patients, with a diagnosis of ovarian epithelial cancer, sEPCR was quantified, blood immune cell phenotypes were determined by flow cytometry and plasma cytokines were evaluated using a protein array. Spearman's rank correlation coefficients (r) and coefficient significance was determined by a statistical hypothesis test (α=0.05). Our results show that i) aPC induced the secretion of several cytokines in Ovcar-3 cells; ii) 61% of patients exhibited a concentration of plasma sEPCR well above the baseline (normal plasma level, 100 ± 28 ng/ml); iii) comparing immune cell phenotypes in patients having a normal level of sEPCR with those having a high level of sEPCR, it was found that sEPCR levels were correlated with high intensity of cells expressing CD45ra, CD3, CD8, CD25 and low intensity of cells expressing CD56 (NK cells), CD294 (TH2 cells), IL-2, IL-10, IL-17a (TH17 cells), IL-21 (TH21 cells) and CD29 markers (r ≥ 0.60); and iv) high levels of sEPCR correlate with high levels of plasma bioactive proteins such as insulin-like growth factor-2 (IGFII), IL-13rα, macrophage inflammatory protein (MIP1α) and matrix metalloproteinase-7 (MMP-7) that have already been proposed as biomarkers for ovarian cancer and particularly those with poor prognosis. In conclusion, sEPCR produced by ovarian cancer cells, by modulating circulating aPC, influences the secretory behavior of tumor cells (cytokines and interleukins). Consequently, sEPCR in turn acts on the innate immune response by decreasing effector cells such as natural killer and T helper cells (TH2, TH17 and TH21).
Figures
References
-
- Dahlbäck B , Villoutreix BO . Molecular recognition in the protein C anticoagulant pathway . J Thromb Haemost . 2003 ; 1 : 1525 – 1534 . - PubMed
-
- Li W , Zheng X , Gu J , Ferrell GL , Lupu F , Esmon NL , Esmon CT . Overexpressing endothelial cell protein C receptor alters the hemostatic balance and protects mice from endotoxin . J Thromb Haemost . 2005 ; 3 : 1351 – 1359 . - PubMed
-
- Taylor FB , Jr , Peer GT , Lockhart MS , Ferrell G , Esmon CT . Endothelial cell protein C receptor plays an important role in protein C activation in vivo . Blood . 2001 ; 97 : 1685 – 1688 . - PubMed
-
- Fukudome K , Kurosawa S , Stearns-Kurosawa DJ , He X , Rezaie AR , Esmon CT . The endothelial cell protein C receptor. Cell surface expression and direct ligand binding by the soluble receptor . J Biol Chem . 1996 ; 271 : 17491 – 17498 . - PubMed
-
- Xu J , Qu D , Esmon NL , Esmon CT . Metalloproteolytic release of endothelial cell protein C receptor . J Biol Chem . 2000 ; 275 : 6038 – 6044 . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

