Control of cell cycle transcription during G1 and S phases
- PMID: 23877564
- PMCID: PMC4569015
- DOI: 10.1038/nrm3629
Control of cell cycle transcription during G1 and S phases
Abstract
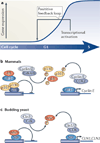
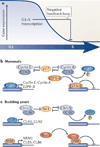
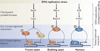
The accurate transition from G1 phase of the cell cycle to S phase is crucial for the control of eukaryotic cell proliferation, and its misregulation promotes oncogenesis. During G1 phase, growth-dependent cyclin-dependent kinase (CDK) activity promotes DNA replication and initiates G1-to-S phase transition. CDK activation initiates a positive feedback loop that further increases CDK activity, and this commits the cell to division by inducing genome-wide transcriptional changes. G1-S transcripts encode proteins that regulate downstream cell cycle events. Recent work is beginning to reveal the complex molecular mechanisms that control the temporal order of transcriptional activation and inactivation, determine distinct functional subgroups of genes and link cell cycle-dependent transcription to DNA replication stress in yeast and mammals.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

