Characterization of Hepatitis C virus resistance from a multiple-dose clinical trial of the novel NS5A inhibitor GS-5885
- PMID: 23877691
- PMCID: PMC3837913
- DOI: 10.1128/AAC.02193-12
Characterization of Hepatitis C virus resistance from a multiple-dose clinical trial of the novel NS5A inhibitor GS-5885
Abstract
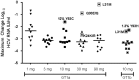
GS-5885 is a novel hepatitis C virus (HCV) NS5A inhibitor. In a 3-day monotherapy study in treatment-naive genotype 1a (GT1a) and GT1b HCV-infected subjects, median viral load reductions ranged from 2.3 to 3.3 log10 HCV RNA IU/ml across dosing cohorts (1, 3, 10, 30, or 90 mg once daily). Here, we report viral sequencing and phenotypic analysis of clinical isolates from this study. Detection of baseline NS5A amino acid substitutions at positions 28, 30, 31, or 93 in GT1a was associated with a reduced treatment response. In the GT1b cohort, Y93H was detected in 100% of subjects at day 4 or 14. In the Gt1a cohort, population sequencing detected NS5A resistance-associated mutations at day 4 or 14 for 3/10 subjects at the 1-mg dose and for all subjects dosed at ≥3 mg. A subset of mutants that confer a low level of reduced susceptibility to GS-5885 was not detected by population sequencing at the 30- and 90-mg doses. Subject-derived M28T, Q30R, L31M, and Y93C mutations all conferred >30-fold reductions in GS-5885 and daclatasvir susceptibilities in vitro. Site-directed NS5A mutants also showed reduced susceptibility to GS-5885. However, all NS5A mutants tested remained fully susceptible to other classes of direct-acting antivirals (DAAs), interferon alpha, and ribavirin. Importantly, the nonoverlapping resistance profile and high potency of GS-5885 support its further development with other direct-acting antivirals for the treatment of chronic HCV. (This study has been registered at ClinicalTrials.gov under registration number NCT01193478.).
Figures

References
-
- Shepard CW, Finelli L, Alter MJ. 2005. Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 5:558–567 - PubMed
-
- Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, George J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda N, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S. 2011. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 364:2405–2416 - PubMed
-
- Gao M, Nettles RE, Belema M, Snyder LB, Nguyen VN, Fridell RA, Serrano-Wu MH, Langley DR, Sun JH, O'Boyle DR, II, Lemm JA, Wang C, Knipe JO, Chien C, Colonno RJ, Grasela DM, Meanwell NA, Hamann LG. 2010. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 465:96–100 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

