P2' benzene carboxylic acid moiety is associated with decrease in cellular uptake: evaluation of novel nonpeptidic HIV-1 protease inhibitors containing P2 bis-tetrahydrofuran moiety
- PMID: 23877703
- PMCID: PMC3811474
- DOI: 10.1128/AAC.00868-13
P2' benzene carboxylic acid moiety is associated with decrease in cellular uptake: evaluation of novel nonpeptidic HIV-1 protease inhibitors containing P2 bis-tetrahydrofuran moiety
Abstract
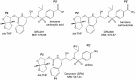
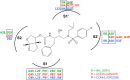
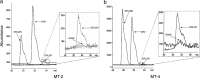
GRL007 and GRL008, two structurally related nonpeptidic human immunodeficiency virus type 1 (HIV-1) protease inhibitors (PIs) containing 3(R),3a(S),6a(R)-bis-tetrahydrofuranylurethane (bis-THF) as the P2 moiety and a sulfonamide isostere consisting of benzene carboxylic acid and benzene carboxamide as the P2' moiety, respectively, were evaluated for their antiviral activity and interactions with wild-type protease (PR(WT)). Both GRL007 (Ki of 12.7 pM with PR(WT)) and GRL008 (Ki of 8.9 pM) inhibited PR(WT) with high potency in vitro. X-ray crystallographic analysis of PR(WT) in complex with GRL007 or GRL008 showed that the bis-THF moiety of both compounds has three direct polar contacts with the backbone amide nitrogen atoms of Asp29 and Asp30 of PR(WT). The P2' moiety of both compounds showed one direct contact with the backbone of Asp30' and a bridging polar contact with Gly48' through a water molecule. Cell-based antiviral assays showed that GRL007 was inactive (50% effective concentration [EC50] of >1 μM) while GRL008 was highly active (EC50 of 0.04 μM) against wild-type HIV-1. High-performance liquid chromatography (HPLC)/mass spectrometry-based cellular uptake assays showed 8.1- and 84-fold higher intracellular concentrations of GRL008 than GRL007 in human MT-2 and MT-4 cell extracts, respectively. Thus, GRL007, in spite of its favorable enzyme-inhibitory activity and protease binding profile, exhibited a lack of antiviral activity in cell-based assays, most likely due to its compromised cellular uptake associated with its P2' benzene carboxylic acid moiety. The anti-HIV-1 potency, favorable toxicity, and binding profile of GRL008 suggest that further optimization of the P2' moiety may improve its antiretroviral features.
Figures


Similar articles
-
GRL-079, a Novel HIV-1 Protease Inhibitor, Is Extremely Potent against Multidrug-Resistant HIV-1 Variants and Has a High Genetic Barrier against the Emergence of Resistant Variants.Antimicrob Agents Chemother. 2018 Apr 26;62(5):e02060-17. doi: 10.1128/AAC.02060-17. Print 2018 May. Antimicrob Agents Chemother. 2018. PMID: 29463535 Free PMC article.
-
A conserved hydrogen-bonding network of P2 bis-tetrahydrofuran-containing HIV-1 protease inhibitors (PIs) with a protease active-site amino acid backbone aids in their activity against PI-resistant HIV.Antimicrob Agents Chemother. 2014 Jul;58(7):3679-88. doi: 10.1128/AAC.00107-14. Epub 2014 Apr 21. Antimicrob Agents Chemother. 2014. PMID: 24752271 Free PMC article.
-
Novel Protease Inhibitors Containing C-5-Modified bis-Tetrahydrofuranylurethane and Aminobenzothiazole as P2 and P2' Ligands That Exert Potent Antiviral Activity against Highly Multidrug-Resistant HIV-1 with a High Genetic Barrier against the Emergence of Drug Resistance.Antimicrob Agents Chemother. 2019 Jul 25;63(8):e00372-19. doi: 10.1128/AAC.00372-19. Print 2019 Aug. Antimicrob Agents Chemother. 2019. PMID: 31085520 Free PMC article.
-
HIV-1 Protease Inhibitors Incorporating Stereochemically Defined P2' Ligands To Optimize Hydrogen Bonding in the Substrate Envelope.J Med Chem. 2019 Sep 12;62(17):8062-8079. doi: 10.1021/acs.jmedchem.9b00838. Epub 2019 Aug 21. J Med Chem. 2019. PMID: 31386368 Free PMC article.
-
Design of HIV protease inhibitors targeting protein backbone: an effective strategy for combating drug resistance.Acc Chem Res. 2008 Jan;41(1):78-86. doi: 10.1021/ar7001232. Epub 2007 Aug 28. Acc Chem Res. 2008. PMID: 17722874 Review.
Cited by
-
GRL-079, a Novel HIV-1 Protease Inhibitor, Is Extremely Potent against Multidrug-Resistant HIV-1 Variants and Has a High Genetic Barrier against the Emergence of Resistant Variants.Antimicrob Agents Chemother. 2018 Apr 26;62(5):e02060-17. doi: 10.1128/AAC.02060-17. Print 2018 May. Antimicrob Agents Chemother. 2018. PMID: 29463535 Free PMC article.
-
A novel central nervous system-penetrating protease inhibitor overcomes human immunodeficiency virus 1 resistance with unprecedented aM to pM potency.Elife. 2017 Oct 17;6:e28020. doi: 10.7554/eLife.28020. Elife. 2017. PMID: 29039736 Free PMC article.
-
Beyond darunavir: recent development of next generation HIV-1 protease inhibitors to combat drug resistance.Chem Commun (Camb). 2022 Oct 20;58(84):11762-11782. doi: 10.1039/d2cc04541a. Chem Commun (Camb). 2022. PMID: 36200462 Free PMC article. Review.
-
Potent HIV-1 Protease Inhibitors Containing Carboxylic and Boronic Acids: Effect on Enzyme Inhibition and Antiviral Activity and Protein-Ligand X-ray Structural Studies.ChemMedChem. 2019 Nov 6;14(21):1863-1872. doi: 10.1002/cmdc.201900508. Epub 2019 Oct 4. ChemMedChem. 2019. PMID: 31549492 Free PMC article.
-
Evaluation of darunavir-derived HIV-1 protease inhibitors incorporating P2' amide-derivatives: Synthesis, biological evaluation and structural studies.Bioorg Med Chem Lett. 2023 Mar 1;83:129168. doi: 10.1016/j.bmcl.2023.129168. Epub 2023 Feb 3. Bioorg Med Chem Lett. 2023. PMID: 36738797 Free PMC article.
References
-
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JHS, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR. 2011. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 365:493–505 - PMC - PubMed
-
- Lalezari JP, Eron JJ, Carlson M, Cohen C, DeJesus E, Arduino RC, Gallant JE, Volberding P, Murphy RL, Valentine F, Nelson EL, Sista PR, Dusek A, Kilby JM. 2003. A phase II clinical study of the long-term safety and antiviral activity of enfuvirtide-based antiretroviral therapy. AIDS 17:691–698 - PubMed
-
- Maeda K, Das D, Nakata H, Mitsuya H. 2012. CCR5 inhibitors: emergence, success, and challenges. Expert Opin. Emerg. Drugs 17:135–145 - PubMed
-
- Mitsuya H, Weinhold KJ, Furman PA, St Clair MH, Lehrman SN, Gallo RC, Bolognesi D, Barry DW, Broder S. 1985. 3′-Azido-3′-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. U. S. A. 82:7096–7100 - PMC - PubMed
-
- Steigbigel RT, Cooper DA, Kumar PN, Eron JE, Schechter M, Markowitz M, Loutfy MR, Lennox JL, Gatell JM, Rockstroh JK, Katlama C, Yeni P, Lazzarin A, Clotet B, Zhao J, Chen J, Ryan DM, Rhodes RR, Killar JA, Gilde LR, Strohmaier KM, Meibohm AR, Miller MD, Hazuda DJ, Nessly ML, DiNubile MJ, Isaacs RD, Nguyen B-Y, Teppler H. 2008. Raltegravir with optimized background therapy for resistant HIV-1 infection. N. Engl. J. Med. 359:339–354 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

