Histone deacetylase AtHDA7 is required for female gametophyte and embryo development in Arabidopsis
- PMID: 23878078
- PMCID: PMC3762662
- DOI: 10.1104/pp.113.221713
Histone deacetylase AtHDA7 is required for female gametophyte and embryo development in Arabidopsis
Abstract
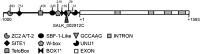
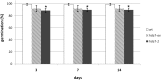
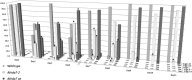
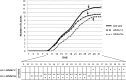
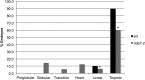
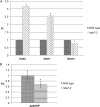
Histone modifications are involved in the regulation of many processes in eukaryotic development. In this work, we provide evidence that AtHDA7, a HISTONE DEACETYLASE (HDAC) of the Reduced Potassium Dependency3 (RPD3) superfamily, is crucial for female gametophyte development and embryogenesis in Arabidopsis (Arabidopsis thaliana). Silencing of AtHDA7 causes degeneration of micropylar nuclei at the stage of four-nucleate embryo sac and delay in the progression of embryo development, thereby bringing the seed set down in the Athda7-2 mutant. Furthermore, AtHDA7 down- and up-regulation lead to a delay of growth in postgermination and later developmental stages. The Athda7-2 mutation that induces histone hyperacetylation significantly increases the transcription of other HDACs (AtHDA6 and AtHDA9). Moreover, silencing of AtHDA7 affects the expression of ARABIDOPSIS HOMOLOG OF SEPARASE (AtAESP), previously demonstrated to be involved in female gametophyte and embryo development. However, chromatin immunoprecipitation analysis with acetylated H3 antibody provided evidence that the acetylation levels of H3 at AtAESP and HDACs does not change in the mutant. Further investigations are essential to ascertain the mechanism by which AtHDA7 affects female gametophyte and embryo development.
Figures




References
-
- Alexander MP. (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44: 117–122 - PubMed
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Barra L, Aiese-Cigliano R, Cremona G, De Luca P, Zoppoli P, Bressan RA, Consiglio MF, Conicella C. (2012) Transcription profiling of laser microdissected microsporocytes in an Arabidopsis mutant (atmcc1) with enhanced histone acetylation. J Plant Biol 55: 281–289
-
- Bell O, Tiwari VK, Thomä NH, Schübeler D. (2011) Determinants and dynamics of genome accessibility. Nat Rev Genet 12: 554–564 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

