Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells
- PMID: 23878230
- PMCID: PMC3740869
- DOI: 10.1073/pnas.1221899110
Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells
Abstract
Recent evidence indicates there is a role for small membrane vesicles, including exosomes, as vehicles for intercellular communication. Exosomes secreted by most cell types can mediate transfer of proteins, mRNAs, and microRNAs, but their role in the transmission of infectious agents is less established. Recent studies have shown that hepatocyte-derived exosomes containing hepatitis C virus (HCV) RNA can activate innate immune cells, but the role of exosomes in the transmission of HCV between hepatocytes remains unknown. In this study, we investigated whether exosomes transfer HCV in the presence of neutralizing antibodies. Purified exosomes isolated from HCV-infected human hepatoma Huh7.5.1 cells were shown to contain full-length viral RNA, viral protein, and particles, as determined by RT-PCR, mass spectrometry, and transmission electron microscopy. Exosomes from HCV-infected cells were capable of transmitting infection to naive human hepatoma Huh7.5.1 cells and establishing a productive infection. Even with subgenomic replicons, lacking structural viral proteins, exosome-mediated transmission of HCV RNA was observed. Treatment with patient-derived IgGs showed a variable degree of neutralization of exosome-mediated infection compared with free virus. In conclusion, this study showed that hepatic exosomes can transmit productive HCV infection in vitro and are partially resistant to antibody neutralization. This discovery sheds light on neutralizing antibodies resistant to HCV transmission by exosomes as a potential immune evasion mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
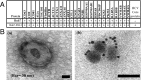


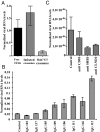
Comment in
-
Hepatitis: New route of HCV transmission.Nat Rev Gastroenterol Hepatol. 2013 Sep;10(9):504. doi: 10.1038/nrgastro.2013.148. Epub 2013 Aug 6. Nat Rev Gastroenterol Hepatol. 2013. PMID: 23917698 No abstract available.
-
HCV transmission by hepatic exosomes establishes a productive infection.J Hepatol. 2014 Mar;60(3):674-5. doi: 10.1016/j.jhep.2013.10.015. Epub 2013 Oct 26. J Hepatol. 2014. PMID: 24512825 No abstract available.
References
-
- Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6(3):267–283. - PubMed
-
- Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. - PubMed
-
- Duijvesz D, Luider T, Bangma CH, Jenster G. Exosomes as biomarker treasure chests for prostate cancer. Eur Urol. 2011;59(5):823–831. - PubMed
-
- Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

