Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy
- PMID: 23878235
- PMCID: PMC3740877
- DOI: 10.1073/pnas.1300910110
Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy
Abstract
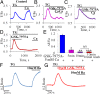
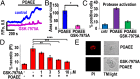
Alcohol-related acute pancreatitis can be mediated by a combination of alcohol and fatty acids (fatty acid ethyl esters) and is initiated by a sustained elevation of the Ca(2+) concentration inside pancreatic acinar cells ([Ca(2+)]i), due to excessive release of Ca(2+) stored inside the cells followed by Ca(2+) entry from the interstitial fluid. The sustained [Ca(2+)]i elevation activates intracellular digestive proenzymes resulting in necrosis and inflammation. We tested the hypothesis that pharmacological blockade of store-operated or Ca(2+) release-activated Ca(2+) channels (CRAC) would prevent sustained elevation of [Ca(2+)]i and therefore protease activation and necrosis. In isolated mouse pancreatic acinar cells, CRAC channels were activated by blocking Ca(2+) ATPase pumps in the endoplasmic reticulum with thapsigargin in the absence of external Ca(2+). Ca(2+) entry then occurred upon admission of Ca(2+) to the extracellular solution. The CRAC channel blocker developed by GlaxoSmithKline, GSK-7975A, inhibited store-operated Ca(2+) entry in a concentration-dependent manner within the range of 1 to 50 μM (IC50 = 3.4 μM), but had little or no effect on the physiological Ca(2+) spiking evoked by acetylcholine or cholecystokinin. Palmitoleic acid ethyl ester (100 μM), an important mediator of alcohol-related pancreatitis, evoked a sustained elevation of [Ca(2+)]i, which was markedly reduced by CRAC blockade. Importantly, the palmitoleic acid ethyl ester-induced trypsin and protease activity as well as necrosis were almost abolished by blocking CRAC channels. There is currently no specific treatment of pancreatitis, but our data show that pharmacological CRAC blockade is highly effective against toxic [Ca(2+)]i elevation, necrosis, and trypsin/protease activity and therefore has potential to effectively treat pancreatitis.
Keywords: AR42J; alcohol metabolite; capacitative Ca2+ entry; hepatocyte Ca2+ entry; pancreas.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: Bench to the bedside. Gastroenterology. 2007;132(3):1127–1151. - PubMed
-
- Petersen OH, Sutton R. Ca2+ signalling and pancreatitis: Effects of alcohol, bile and coffee. Trends Pharmacol Sci. 2006;27(2):113–120. - PubMed
-
- Petersen OH, et al. Fatty acids, alcohol and fatty acid ethyl esters: Toxic Ca2+ signal generation and pancreatitis. Cell Calcium. 2009;45(6):634–642. - PubMed
-
- Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. 2008;70:273–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

