Higd-1a interacts with Opa1 and is required for the morphological and functional integrity of mitochondria
- PMID: 23878241
- PMCID: PMC3740888
- DOI: 10.1073/pnas.1307170110
Higd-1a interacts with Opa1 and is required for the morphological and functional integrity of mitochondria
Abstract
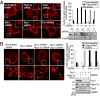
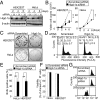
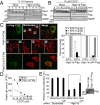
The activity and morphology of mitochondria are maintained by dynamic fusion and fission processes regulated by a group of proteins residing in, or attached to, their inner and outer membranes. Hypoxia-induced gene domain protein-1a (Higd-1a)/HIMP1-a/HIG1, a mitochondrial inner membrane protein, plays a role in cell survival under hypoxic conditions. In the present study, we showed that Higd-1a depletion resulted in mitochondrial fission, depletion of mtDNA, disorganization of cristae, and growth retardation. We demonstrated that Higd-1a functions by specifically binding to Optic atrophy 1 (Opa1), a key element in fusion of the inner membrane. In the absence of Higd-1a, Opa1 was cleaved, resulting in the loss of its long isoforms and accumulation of small soluble forms. The small forms of Opa1 do not interact with Higd-1a, suggesting that a part of Opa1 in or proximal to the membrane is required for that interaction. Opa1 cleavage, mitochondrial fission, and cell death induced by dissipation of the mitochondrial membrane potential were significantly inhibited by ectopic expression of Higd-1a. Furthermore, growth inhibition due to Higd-1a depletion could be overcome by overexpression of a noncleavable form of Opa1. Collectively, our observations demonstrate that Higd-1a inhibits Opa1 cleavage and is required for mitochondrial fusion by virtue of its interaction with Opa1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. - PubMed
-
- Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114(Pt 5):867–874. - PubMed
-
- Rojo M, Legros F, Chateau D, Lombès A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci. 2002;115(Pt 8):1663–1674. - PubMed
-
- Koshiba T, et al. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305(5685):858–862. - PubMed
-
- Olichon A, et al. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 2002;523(1-3):171–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

