Shear stress activation of nuclear receptor PXR in endothelial detoxification
- PMID: 23878263
- PMCID: PMC3740860
- DOI: 10.1073/pnas.1312065110
Shear stress activation of nuclear receptor PXR in endothelial detoxification
Abstract
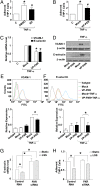
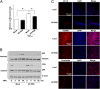
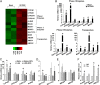
Endothelial cells (ECs) are constantly exposed to xenobiotics and endobiotics or their metabolites, which perturb EC function, as well as to shear stress, which plays a crucial role in vascular homeostasis. Pregnane X receptor (PXR) is a nuclear receptor and a key regulator of the detoxification of xeno- and endobiotics. Here we show that laminar shear stress (LSS), the atheroprotective flow, activates PXR in ECs, whereas oscillatory shear stress, the atheroprone flow, suppresses PXR. LSS activation of PXR in cultured ECs led to the increased expression of a PXR target gene, multidrug resistance 1 (MDR1). An in vivo study using rats showed that the expression of MDR1 was significantly higher in the endothelium from the descending thoracic aorta, where flow is mostly laminar, than from the inner curvature of aortic arch, where flow is disturbed. Functionally, LSS-activated PXR protects ECs from apoptosis triggered by doxorubicin via the induction of MDR1 and other detoxification genes. PXR also suppressed the expression of proinflammatory adhesion molecules and monocyte adhesion in response to TNF-α and lipopolysaccharide. Overexpression of a constitutively active PXR in rat carotid arteries potently attenuated proinflammatory responses. In addition, cDNA microarray revealed a large number of the PXR-activated endothelial genes whose products are responsible for major steps of detoxification, including phase I and II metabolizing enzymes and transporters. These detoxification genes in ECs are induced by LSS in ECs in a PXR-dependent manner. In conclusion, our results indicate that PXR represents a flow-activated detoxification system to protect ECs against damage by xeno- and endobiotics.
Keywords: endothelial homeostasis; gene regulation; hemodynamics; nuclear hormone receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Ginkgolide B protects human umbilical vein endothelial cells against xenobiotic injuries via PXR activation.Acta Pharmacol Sin. 2016 Feb;37(2):177-86. doi: 10.1038/aps.2015.124. Epub 2016 Jan 18. Acta Pharmacol Sin. 2016. PMID: 26775663 Free PMC article.
-
Xenobiotic pregnane X receptor (PXR) regulates innate immunity via activation of NLRP3 inflammasome in vascular endothelial cells.J Biol Chem. 2014 Oct 24;289(43):30075-81. doi: 10.1074/jbc.M114.578781. Epub 2014 Sep 8. J Biol Chem. 2014. PMID: 25202020 Free PMC article.
-
Pregnane X receptor regulates drug metabolism and transport in the vasculature and protects from oxidative stress.Cardiovasc Res. 2012 Mar 15;93(4):674-81. doi: 10.1093/cvr/cvr330. Epub 2011 Dec 13. Cardiovasc Res. 2012. PMID: 22166712 Free PMC article.
-
The nuclear pregnane X receptor regulates xenobiotic detoxification.J Nutr. 2003 Jul;133(7 Suppl):2444S-2447S. doi: 10.1093/jn/133.7.2444S. J Nutr. 2003. PMID: 12840222 Review.
-
Induction of phase I, II and III drug metabolism/transport by xenobiotics.Arch Pharm Res. 2005 Mar;28(3):249-68. doi: 10.1007/BF02977789. Arch Pharm Res. 2005. PMID: 15832810 Review.
Cited by
-
Xenobiotic Receptor-Mediated Regulation of Intestinal Barrier Function and Innate Immunity.Nucl Receptor Res. 2016;3:101199. doi: 10.11131/2016/101199. Nucl Receptor Res. 2016. PMID: 27942535 Free PMC article.
-
Lack of CAR impacts neuronal function and cerebrovascular integrity in vivo.Exp Neurol. 2016 Sep;283(Pt A):39-48. doi: 10.1016/j.expneurol.2016.05.018. Epub 2016 May 27. Exp Neurol. 2016. PMID: 27240521 Free PMC article.
-
Marine Ligands of the Pregnane X Receptor (PXR): An Overview.Mar Drugs. 2019 Sep 28;17(10):554. doi: 10.3390/md17100554. Mar Drugs. 2019. PMID: 31569349 Free PMC article. Review.
-
Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications.Front Med (Lausanne). 2022 Jan 20;8:798958. doi: 10.3389/fmed.2021.798958. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35127755 Free PMC article. Review.
-
The pregnane X receptor and its microbiota-derived ligand indole 3-propionic acid regulate endothelium-dependent vasodilation.Am J Physiol Endocrinol Metab. 2019 Aug 1;317(2):E350-E361. doi: 10.1152/ajpendo.00572.2018. Epub 2019 Jun 18. Am J Physiol Endocrinol Metab. 2019. PMID: 31211619 Free PMC article.
References
-
- Dimmeler S, Haendeler J, Rippmann V, Nehls M, Zeiher AM. Shear stress inhibits apoptosis of human endothelial cells. FEBS Lett. 1996;399(1-2):71–74. - PubMed
-
- Dimmeler S, Hermann C, Galle J, Zeiher AM. Upregulation of superoxide dismutase and nitric oxide synthase mediates the apoptosis-suppressive effects of shear stress on endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19(3):656–664. - PubMed
-
- Hermann C, Zeiher AM, Dimmeler S. Shear stress inhibits H2O2-induced apoptosis of human endothelial cells by modulation of the glutathione redox cycle and nitric oxide synthase. Arterioscler Thromb Vasc Biol. 1997;17(12):3588–3592. - PubMed
-
- Tricot O, et al. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000;101(21):2450–2453. - PubMed
-
- Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7(5):584–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

