Coordinated waves of actomyosin flow and apical cell constriction immediately after wounding
- PMID: 23878279
- PMCID: PMC3718973
- DOI: 10.1083/jcb.201211039
Coordinated waves of actomyosin flow and apical cell constriction immediately after wounding
Abstract
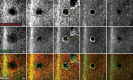
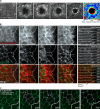
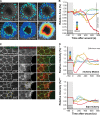
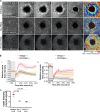
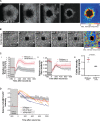
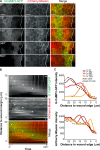
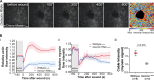
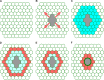
Epithelial wound healing relies on tissue movements and cell shape changes. Our work shows that, immediately after wounding, there was a dramatic cytoskeleton remodeling consisting of a pulse of actomyosin filaments that assembled in cells around the wound edge and flowed from cell to cell toward the margin of the wound. We show that this actomyosin flow was regulated by Diaphanous and ROCK and that it elicited a wave of apical cell constriction that culminated in the formation of the leading edge actomyosin cable, a structure that is essential for wound closure. Calcium signaling played an important role in this process, as its intracellular concentration increased dramatically immediately after wounding, and down-regulation of transient receptor potential channel M, a stress-activated calcium channel, also impaired the actomyosin flow. Lowering the activity of Gelsolin, a known calcium-activated actin filament-severing protein, also impaired the wound response, indicating that cleaving the existing actin filament network is an important part of the cytoskeleton remodeling process.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

