Vaccine-induced gag-specific T cells are associated with reduced viremia after HIV-1 infection
- PMID: 23878319
- PMCID: PMC3778967
- DOI: 10.1093/infdis/jit322
Vaccine-induced gag-specific T cells are associated with reduced viremia after HIV-1 infection
Abstract
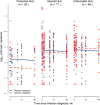
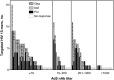
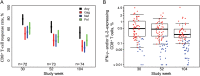
The contribution of host T-cell immunity and HLA class I alleles to the control of human immunodeficiency virus (HIV-1) replication in natural infection is widely recognized. We assessed whether vaccine-induced T-cell immunity, or expression of certain HLA alleles, impacted HIV-1 control after infection in the Step MRKAd5/HIV-1 gag/pol/nef study. Vaccine-induced T cells were associated with reduced plasma viremia, with subjects targeting ≥3 gag peptides presenting with half-log lower mean viral loads than subjects without Gag responses. This effect was stronger in participants infected proximal to vaccination and was independent of our observed association of HLA-B*27, -B*57 and -B*58:01 alleles with lower HIV-1 viremia. These findings support the ability of vaccine-induced T-cell responses to influence postinfection outcome and provide a rationale for the generation of T-cell responses by vaccination to reduce viremia if protection from acquisition is not achieved. Clinical trials identifier: NCT00095576.
Keywords: Gag-specific T cells; HIV-1 vaccine; HLA class I alleles; Step study.
Figures

References
-
- Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–56. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

