Platelet-derived growth factor/vascular endothelial growth factor receptor inactivation by sunitinib results in Tsc1/Tsc2-dependent inhibition of TORC1
- PMID: 23878397
- PMCID: PMC3811881
- DOI: 10.1128/MCB.01570-12
Platelet-derived growth factor/vascular endothelial growth factor receptor inactivation by sunitinib results in Tsc1/Tsc2-dependent inhibition of TORC1
Abstract
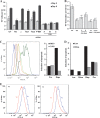
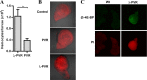
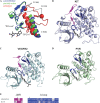
Vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) receptors are implicated in development and tumorigenesis and dual inhibitors like sunitinib are prescribed for cancer treatment. While mammalian VEGF and PDGF receptors are present in multiple isoforms and heterodimers, Drosophila encodes one ancestral PDGF/VEGF receptor, PVR. We identified PVR in an unbiased cell-based RNA interference (RNAi) screen of all Drosophila kinases and phosphatases for novel regulators of TORC1. PVR is essential to sustain target of rapamycin complex 1 (TORC1) and extracellular signal-regulated kinase (ERK) activity in cultured insect cells and for maximal stimulation by insulin. CG32406 (henceforth, PVRAP, for PVR adaptor protein), an Src homology 2 (SH2) domain-containing protein, binds PVR and is required for TORC1 activation. TORC1 activation by PVR involves Tsc1/Tsc2 and, in a cell-type-dependent manner, Lobe (ortholog of PRAS40). PVR is required for cell survival in vitro, and both PVR and TORC1 are necessary for hemocyte expansion in vivo. Constitutive PVR activation induces tumor-like structures that exhibit high TORC1 activity. Like its mammalian orthologs, PVR is inhibited by sunitinib, and sunitinib treatment phenocopies PVR loss in hemocytes. Sunitinib inhibits TORC1 in insect cells, and sunitinib-mediated TORC1 inhibition requires an intact Tsc1/Tsc2 complex. Sunitinib similarly inhibited TORC1 in human endothelial cells in a Tsc1/Tsc2-dependent manner. Our findings provide insight into the mechanism of action of PVR and may have implications for understanding sunitinib sensitivity and resistance in tumors.
Figures












References
-
- Hoch RV, Soriano P. 2003. Roles of PDGF in animal development. Development 130:4769–4784 - PubMed
-
- Heldin CH, Westermark B. 1999. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 79:1283–1316 - PubMed
-
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. 2006. VEGF receptor signalling—in control of vascular function. Nat. Rev. Mol. Cell Biol. 7:359–371 - PubMed
-
- Ferrara N, Gerber HP, LeCouter J. 2003. The biology of VEGF and its receptors. Nat. Med. 9:669–676 - PubMed
-
- Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. 2003. VEGF-receptor signal transduction. Trends Biochem. Sci. 28:488–494 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
