Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation
- PMID: 23878739
- PMCID: PMC3708441
- DOI: 10.1155/2013/752864
Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation
Abstract
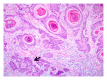
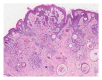
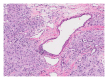
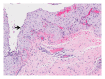
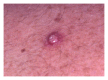
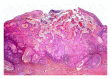
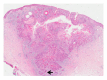
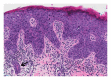
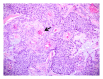
Squamous cell carcinoma (SCC) is a common and important primary cutaneous malignancy. On skin biopsies, SCC is characterized by significant squamous cell atypia, abnormal keratinization, and invasive features. Diagnostic challenges may occasionally arise, especially in the setting of small punch biopsies or superficial shave biopsies, where only part of the lesion may be assessable by the pathologist. Benign mimics of SCC include pseudoepitheliomatous hyperplasia, eccrine squamous syringometaplasia, inverted follicular keratosis, and keratoacanthoma, while malignant mimics of SCC include basal cell carcinoma, melanoma, and metastatic carcinoma. The careful application of time-honored diagnostic criteria, close clinicopathological correlation and a selective request for a further, deeper, or wider biopsy remain the most useful strategies to clinch the correct diagnosis. This review aims to present the key differential diagnoses of SCC, to discuss common diagnostic pitfalls, and to recommend ways to deal with diagnostically challenging cases.
Figures







Similar articles
-
Pseudoepitheliomatous hyperplasia in lichen sclerosus of the vulva.Int J Gynecol Pathol. 2003 Jan;22(1):57-62. doi: 10.1097/00004347-200301000-00012. Int J Gynecol Pathol. 2003. PMID: 12496699
-
Diagnostic value of CD10 and Bcl2 expression in distinguishing cutaneous basal cell carcinoma from squamous cell carcinoma and seborrheic keratosis.Pathol Res Pract. 2015 Dec;211(12):931-8. doi: 10.1016/j.prp.2015.09.009. Epub 2015 Oct 9. Pathol Res Pract. 2015. PMID: 26573127
-
Histopathological diagnosis of epithelial crateriform tumors: Keratoacanthoma and other epithelial crateriform tumors.J Dermatol. 2016 Nov;43(11):1321-1331. doi: 10.1111/1346-8138.13390. Epub 2016 Apr 14. J Dermatol. 2016. PMID: 27076258
-
Keratoacanthoma: hyperplasia, benign neoplasm, or a type of squamous cell carcinoma?Semin Diagn Pathol. 2009 Aug;26(3):150-63. doi: 10.1053/j.semdp.2009.09.003. Semin Diagn Pathol. 2009. PMID: 20043514 Review.
-
Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification--part two.J Cutan Pathol. 2006 Apr;33(4):261-79. doi: 10.1111/j.0303-6987.2006.00516.x. J Cutan Pathol. 2006. PMID: 16630176 Review.
Cited by
-
A verrucous lesion of the eyebrow.Indian Dermatol Online J. 2016 May-Jun;7(3):206-7. doi: 10.4103/2229-5178.182370. Indian Dermatol Online J. 2016. PMID: 27294062 Free PMC article. No abstract available.
-
Granuloma Annulare Mimicking Squamous Cell Carcinoma.Cureus. 2022 Jul 27;14(7):e27372. doi: 10.7759/cureus.27372. eCollection 2022 Jul. Cureus. 2022. PMID: 36046278 Free PMC article.
-
Cytokeratin 17 and Ki-67: Immunohistochemical markers for the differential diagnosis of keratoacanthoma and squamous cell carcinoma.Oncol Lett. 2017 Apr;13(4):2539-2548. doi: 10.3892/ol.2017.5793. Epub 2017 Mar 1. Oncol Lett. 2017. PMID: 28454431 Free PMC article.
-
Pseudoepitheliomatous hyperplasia: a clinical entity mistaken for squamous cell carcinoma.J Cutan Aesthet Surg. 2014 Oct-Dec;7(4):232-4. doi: 10.4103/0974-2077.150787. J Cutan Aesthet Surg. 2014. PMID: 25722605 Free PMC article. No abstract available.
-
Cutaneous Squamous Cell Carcinoma Mimickers: Beware the Pruritic Papule on the Leg of an Older Female.Cureus. 2023 Jul 8;15(7):e41569. doi: 10.7759/cureus.41569. eCollection 2023 Jul. Cureus. 2023. PMID: 37554602 Free PMC article.
References
-
- Diepgen TL, Mahler V. The epidemiology of skin cancer. British Journal of Dermatology. 2002;146(61) Supplement:1–6. - PubMed
-
- Swanson PE, Fitzpatrick MM, Ritter JH, Glusac EJ, Wick MR. Immunohistologic differential diagnosis of basal cell carcinoma, squamous cell carcinoma, and trichoepithelioma in small cutaneous biopsy specimens. Journal of Cutaneous Pathology. 1998;25(3):153–159. - PubMed
-
- Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline: update 2012. European Journal of Cancer. 2012;48(15):2375–2390. - PubMed
-
- Schrama D, Ugurel S, Becker JC. Merkel cell carcinoma: recent insights and new treatment options. Current Opinion in Oncology. 2012;24(2):141–149. - PubMed
-
- Mehregan AH. Inverted follicular keratosis is a distinct follicular tumor. The American Journal of Dermatopathology. 1983;5(5):467–470. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

