Plant growth promotion potential is equally represented in diverse grapevine root-associated bacterial communities from different biopedoclimatic environments
- PMID: 23878810
- PMCID: PMC3708380
- DOI: 10.1155/2013/491091
Plant growth promotion potential is equally represented in diverse grapevine root-associated bacterial communities from different biopedoclimatic environments
Abstract
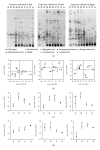
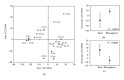
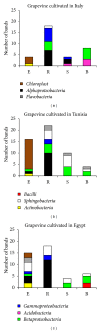
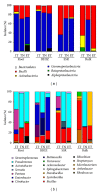
Plant-associated bacteria provide important services to host plants. Environmental factors such as cultivar type and pedoclimatic conditions contribute to shape their diversity. However, whether these environmental factors may influence the plant growth promoting (PGP) potential of the root-associated bacteria is not widely understood. To address this issue, the diversity and PGP potential of the bacterial assemblage associated with the grapevine root system of different cultivars in three Mediterranean environments along a macrotransect identifying an aridity gradient were assessed by culture-dependent and independent approaches. According to 16S rRNA gene PCR-DGGE, the structure of endosphere and rhizosphere bacterial communities was highly diverse (P = 0.03) and was associated with a cultivar/latitudinal/climatic effect. Despite being diverse, the bacterial communities associated with Egyptian grapevines shared a higher similarity with the Tunisian grapevines than those cultivated in North Italy. A similar distribution, according to the cultivar/latitude/aridity gradients, was observed for the cultivable bacteria. Many isolates (23%) presented in vitro multiple stress resistance capabilities and PGP activities, the most frequent being auxin synthesis (82%), insoluble phosphate solubilisation (61%), and ammonia production (70%). The comparable numbers and types of potential PGP traits among the three different environmental settings indicate a strong functional homeostasis of beneficial bacteria associated with grape root.
Figures
Similar articles
-
Grapevine rootstocks shape underground bacterial microbiome and networking but not potential functionality.Microbiome. 2018 Jan 3;6(1):3. doi: 10.1186/s40168-017-0391-2. Microbiome. 2018. PMID: 29298729 Free PMC article.
-
The date palm tree rhizosphere is a niche for plant growth promoting bacteria in the oasis ecosystem.Biomed Res Int. 2015;2015:153851. doi: 10.1155/2015/153851. Epub 2015 Mar 19. Biomed Res Int. 2015. PMID: 25866759 Free PMC article.
-
Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait.Environ Microbiol. 2015 Feb;17(2):316-31. doi: 10.1111/1462-2920.12439. Epub 2014 Mar 25. Environ Microbiol. 2015. PMID: 24571749
-
Diversity of bacterial endophytes in 3 and 15 year-old grapevines of Vitis vinifera cv. Corvina and their potential for plant growth promotion and phytopathogen control.Microbiol Res. 2016 Feb;183:42-52. doi: 10.1016/j.micres.2015.11.009. Epub 2015 Nov 25. Microbiol Res. 2016. PMID: 26805617
-
Beneficial Bacteria Isolated from Grapevine Inner Tissues Shape Arabidopsis thaliana Roots.PLoS One. 2015 Oct 16;10(10):e0140252. doi: 10.1371/journal.pone.0140252. eCollection 2015. PLoS One. 2015. PMID: 26473358 Free PMC article.
Cited by
-
Rhizobacterial communities of five co-occurring desert halophytes.PeerJ. 2018 Aug 30;6:e5508. doi: 10.7717/peerj.5508. eCollection 2018. PeerJ. 2018. PMID: 30186688 Free PMC article.
-
Isolation and characterization of a hydrocarbonoclastic bacterial enrichment from total petroleum hydrocarbon contaminated sediments: potential candidates for bioaugmentation in bio-based processes.Environ Sci Pollut Res Int. 2016 Jun;23(11):10587-10594. doi: 10.1007/s11356-015-5944-y. Epub 2016 Jan 12. Environ Sci Pollut Res Int. 2016. PMID: 26755178
-
The microbiota of the grapevine holobiont: A key component of plant health.J Adv Res. 2022 Sep;40:1-15. doi: 10.1016/j.jare.2021.12.008. Epub 2021 Dec 22. J Adv Res. 2022. PMID: 36100319 Free PMC article. Review.
-
Are drought-resistance promoting bacteria cross-compatible with different plant models?Plant Signal Behav. 2013 Oct;8(10):e26741. doi: 10.4161/psb.26741. Plant Signal Behav. 2013. PMID: 24270625 Free PMC article.
-
Salicornia strobilacea (Synonym of Halocnemum strobilaceum) Grown under Different Tidal Regimes Selects Rhizosphere Bacteria Capable of Promoting Plant Growth.Front Microbiol. 2016 Aug 22;7:1286. doi: 10.3389/fmicb.2016.01286. eCollection 2016. Front Microbiol. 2016. PMID: 27597846 Free PMC article.
References
-
- Shi XY, Bi JL, Morse JG, Toscano NC, Cooksey DA. Effect of xylem fluid from susceptible and resistant grapevines on developmental biology of Xylella fastidiosa . European Journal of Plant Pathology. 2013;135:127–135.
-
- Aballay E, Mårtensson A, Persson P. Screening of rhizosphere bacteria from grapevine for their suppressive effect on Xiphinema index Thorne & Allen on in vitro grape plants. Plant and Soil. 2011;347(1):313–325.
-
- Balloi A, Rolli E, Marasco R, et al. The role of microorganisms in bioremediation and phytoremediation of polluted and stressed soils. Agrochimica. 2010;54(6):353–369.
-
- Compant S, Mitter B, Colli-Mull JG, Gangl H, Sessitsch A. Endophytes of grapevine flowers, berries, and eeds: identification of cultivable bacteria, comparison with other plant parts, and visualization of niches of colonization. Microbial Ecology. 2011;62(1):188–197. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

