Nickel-catalyzed Suzuki-Miyaura couplings in green solvents
- PMID: 23879392
- PMCID: PMC3768281
- DOI: 10.1021/ol401727y
Nickel-catalyzed Suzuki-Miyaura couplings in green solvents
Abstract
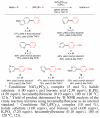
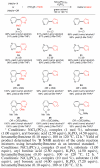
The nickel-catalyzed Suzuki-Miyaura coupling of aryl halides and phenol-derived substrates with aryl boronic acids using green solvents, such as 2-Me-THF and tert-amyl alcohol, is reported. This methodology employs the commercially available and air-stable precatalyst, NiCl2(PCy3)2, and gives biaryl products in synthetically useful to excellent yields. Using this protocol, bis(heterocyclic) frameworks can be assembled efficiently.
Figures



References
-
- Diederich F, Meijere A, editors. Metal-Catalyzed Cross-Coupling Reactions. Wiley-VCH; Weinheim: 2004.
- Hassan J, Sevignon M, Gozzi C, Schulz E, Lemaire M. Chem. Rev. 2002;102:1359–1469. - PubMed
- Miyaura N, editor. Topics in Current Chemistr. Vol. 219. Springer-Verlag; New York: 2002.
- Corbet J, Mignani G. Chem. Rev. 2006;106:2651–2710. - PubMed
- Negishi E. Bull. Chem. Soc. Jpn. 2007;80:233–257.
- Shen HC, Crawley ML, Trost BM, editors. Application of Transition Metal Catalysis in Drug Discovery and Development: An Industrial Perspective. John Wiley & Sons, Inc.; Hoboken: 2012.
-
-
For solvent selection guide in medicinal chemistry, see: Alfonsi K, Colberg J, Dunn PJ, Fevig T, Jennings S, Johnson TA, Kleine HP, Knight C, Nagy MA, Perry DA, Stefaniak M. Green Chem. 2008;10:31–36.. Henderson RK, Jiménez-Gonález C, Constable DJC, Alston SR, Inglis GGA, Fisher G, Sherwood J, Binks SP, Curzons AD. Green Chem. 2011;13:854–862.
-
-
-
For examples of cross-coupling reactions in aqueous media, see Bussolari JC, Rehborn DC. Org. Lett. 1999;1:965–967.. Hesse S, Kirsch G. Synthesis. 2001:755–759.. Anderson KW, Buchwald SL. Angew. Chem. Int. Ed. 2005;44:6173–6177.. Lipshutz BH, Ghorai S. Aldrichim. Acta. 2008;41:59–72.. Alacid E, Nájera C. J. Org. Chem. 2009;74:2321–2327.. Han J, Liu Y, Guo R. J. Am. Chem. Soc. 2009;131:2060–2061.. Chalker JM, Wood CS, Davis BG. J. Am. Chem. Soc. 2009;131:16346–16347..
-
-
- Anastas PT, Warner JC, editors. Green Chemistry: Theory and Practice. Oxford University Press; New York: 1998.
- Anastas PT, Kirchhoff MM. Acc. Chem. Res. 2002;35:686–694. - PubMed
-
- Fortunak JM. Future Med. Chem. 2009;1:571–575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

