Controlled cortical impact results in an extensive loss of dendritic spines that is not mediated by injury-induced amyloid-beta accumulation
- PMID: 23879560
- PMCID: PMC3837436
- DOI: 10.1089/neu.2013.2960
Controlled cortical impact results in an extensive loss of dendritic spines that is not mediated by injury-induced amyloid-beta accumulation
Abstract
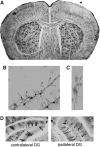
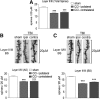
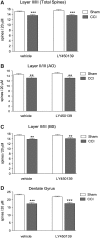
The clinical manifestations that occur after traumatic brain injury (TBI) include a wide range of cognitive, emotional, and behavioral deficits. The loss of excitatory synapses could potentially explain why such diverse symptoms occur after TBI, and a recent preclinical study has demonstrated a loss of dendritic spines, the postsynaptic site of the excitatory synapse, after fluid percussion injury. The objective of this study was to determine if controlled cortical impact (CCI) also resulted in dendritic spine retraction and to probe the underlying mechanisms of this spine loss. We used a unilateral CCI and visualized neurons and dendtritic spines at 24 h post-injury using Golgi stain. We found that TBI caused a 32% reduction of dendritic spines in layer II/III of the ipsilateral cortex and a 20% reduction in the dendritic spines of the ipsilateral dentate gyrus. Spine loss was not restricted to the ipsilateral hemisphere, however, with similar reductions in spine numbers recorded in the contralateral cortex (25% reduction) and hippocampus (23% reduction). Amyloid-β (Aβ), a neurotoxic peptide commonly associated with Alzheimer disease, accumulates rapidly after TBI and is also known to cause synaptic loss. To determine if Aβ contributes to spine loss after brain injury, we administered a γ-secretase inhibitor LY450139 after TBI. We found that while LY450139 administration could attenuate the TBI-induced increase in Aβ, it had no effect on dendritic spine loss after TBI. We conclude that the acute, global loss of dendritic spines after TBI is independent of γ-secretase activity or TBI-induced Aβ accumulation.
Figures


Similar articles
-
Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus.PLoS One. 2011;6(9):e24566. doi: 10.1371/journal.pone.0024566. Epub 2011 Sep 13. PLoS One. 2011. PMID: 21931758 Free PMC article.
-
MMP-9 Contributes to Dendritic Spine Remodeling Following Traumatic Brain Injury.Neural Plast. 2019 May 6;2019:3259295. doi: 10.1155/2019/3259295. eCollection 2019. Neural Plast. 2019. PMID: 31198417 Free PMC article.
-
Traumatic brain injury causes an FK506-sensitive loss and an overgrowth of dendritic spines in rat forebrain.J Neurotrauma. 2012 Jan 20;29(2):201-17. doi: 10.1089/neu.2011.1761. J Neurotrauma. 2012. PMID: 21517673
-
Dendritic spine loss and synaptic alterations in Alzheimer's disease.Mol Neurobiol. 2008 Feb;37(1):73-82. doi: 10.1007/s12035-008-8018-z. Epub 2008 Apr 26. Mol Neurobiol. 2008. PMID: 18438727 Review.
-
Synapses and dendritic spines as pathogenic targets in Alzheimer's disease.Neural Plast. 2012;2012:247150. doi: 10.1155/2012/247150. Epub 2012 Feb 6. Neural Plast. 2012. PMID: 22474602 Free PMC article. Review.
Cited by
-
Impact of Repetitive Mild Traumatic Brain Injury on Behavioral and Hippocampal Deficits in a Mouse Model of Chronic Stress.J Neurotrauma. 2019 Sep 1;36(17):2590-2607. doi: 10.1089/neu.2018.6314. Epub 2019 May 23. J Neurotrauma. 2019. PMID: 30963958 Free PMC article.
-
Mapping the Connectome Following Traumatic Brain Injury.Curr Neurol Neurosci Rep. 2016 May;16(5):44. doi: 10.1007/s11910-016-0642-9. Curr Neurol Neurosci Rep. 2016. PMID: 27021773 Review.
-
Blockade of Astrocytic Calcineurin/NFAT Signaling Helps to Normalize Hippocampal Synaptic Function and Plasticity in a Rat Model of Traumatic Brain Injury.J Neurosci. 2016 Feb 3;36(5):1502-15. doi: 10.1523/JNEUROSCI.1930-15.2016. J Neurosci. 2016. PMID: 26843634 Free PMC article.
-
Bumetanide Prevents Brain Trauma-Induced Depressive-Like Behavior.Front Mol Neurosci. 2019 Feb 5;12:12. doi: 10.3389/fnmol.2019.00012. eCollection 2019. Front Mol Neurosci. 2019. PMID: 30804751 Free PMC article.
-
Impact of amyloid-beta changes on cognitive outcomes in Alzheimer's disease: analysis of clinical trials using a quantitative systems pharmacology model.Alzheimers Res Ther. 2018 Feb 2;10(1):14. doi: 10.1186/s13195-018-0343-5. Alzheimers Res Ther. 2018. PMID: 29394903 Free PMC article.
References
-
- Jamora C.W. Young A. Ruff R.M. Comparison of subjective cognitive complaints with neuropsychological tests in individuals with mild vs more severe traumatic brain injuries. Brain Inj. 2012;26:36–47. - PubMed
-
- Rapoport M. McCauley S. Levin H. Song J. Feinstein A. The role of injury severity in neurobehavioral outcome 3 months after traumatic brain injury. Neuropsychiatry Neuropsychol. Behav. Neurol. 2002;15:123–132. - PubMed
-
- Satz P. Zaucha K. Forney D.L. McCleary C. Asarnow R.F. Light R. Levin H. Kelly D. Bergsneider M. Hovda D. Martin N. Caron M.J. Namerow N. Becker D. Neuropsychological, psychosocial and vocational correlates of the Glasgow Outcome Scale at 6 months post-injury: a study of moderate to severe traumatic brain injury patients. Brain Inj. 1998;12:555–567. - PubMed
-
- Catala I. Ferrer I. Galofre E. Fabregues I. Decreased numbers of dendritic spines on cortical pyramidal neurons in dementia. A quantitative Golgi study on biopsy samples. Hum.Neurobiol. 1988;6:255–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

