Homologous lactoferrin triggers mobilization of the myelocytic lineage of bone marrow in experimental mice
- PMID: 23879888
- PMCID: PMC3868385
- DOI: 10.1089/scd.2013.0242
Homologous lactoferrin triggers mobilization of the myelocytic lineage of bone marrow in experimental mice
Abstract
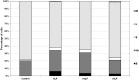
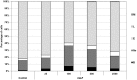
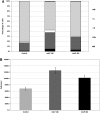
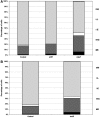
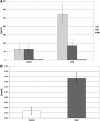
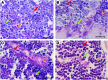
The effects of lactoferrin (LF), an iron binding protein, on myelopoiesis have been studied extensively in vitro and in vivo in human and murine models over the past three decades. Due to the lack of high-quality homologous LFs, however, the conclusions are still unequivocal. Recently, both human and murine LFs have become available as recombinant products expressed in Chinese hamster ovary (CHO) cell lines showing mammalian type of glycosylation, thus apparently species compatible. In this study, we present the effects of homologous recombinant mouse LF (rmLF) on myelopoiesis in CBA mice. The myelocytic lineage has been assessed by their appearance in circulating blood and bone marrow, and induction of relevant mediators of inflammation. Intravenous injection of rmLF (100 μg/mouse) resulted in a significantly increased number of myelocytic cells in the circulating blood after 24 h. Mouse serum transferrin, used as a control protein, showed no stimulatory effect. The increase in output of neutrophil precursors, neutrophils, and eosinophils was correlated with a twofold increase of leukocyte concentrations. The analysis of the bone marrow sections confirmed increased myelopoiesis. The alterations in the bone marrow cell composition were statistically significant regarding mature neutrophils (10.8% vs. 27.7%), metamyelocytes (11.4% vs. 16.0%), and myelocytes (2.4% vs. 4.0%). The mobilization of the myelocytic cells in the bone marrow and the increased output of these cells into circulation were accompanied by elevated serum concentrations of interleukin-6 at 6 h and haptoglobin at 24 h following administration of rmLF. In conclusion, the homologous LF elicits significant and transient myelopoiesis in experimental mice.
Figures


Similar articles
-
Endogenous steroids are responsible for lactoferrin-induced myelopoiesis in mice.Pharmacol Rep. 2009 Jul-Aug;61(4):705-10. doi: 10.1016/s1734-1140(09)70123-9. Pharmacol Rep. 2009. PMID: 19815953
-
Lactoferrin accelerates reconstitution of the humoral and cellular immune response during chemotherapy-induced immunosuppression and bone marrow transplant in mice.Stem Cells Dev. 2005 Oct;14(5):548-55. doi: 10.1089/scd.2005.14.548. Stem Cells Dev. 2005. PMID: 16305339
-
Protective effects of lactoferrin in Escherichia coli-induced bacteremia in mice: relationship to reduced serum TNF alpha level and increased turnover of neutrophils.Inflamm Res. 2004 Jul;53(7):292-6. doi: 10.1007/s00011-004-1257-1. Epub 2004 Jun 25. Inflamm Res. 2004. PMID: 15241563
-
Genomic expression during human myelopoiesis.BMC Genomics. 2007 Aug 3;8:264. doi: 10.1186/1471-2164-8-264. BMC Genomics. 2007. PMID: 17683550 Free PMC article.
-
The effects of lactoferrin on myelopoiesis: can we resolve the controversy?Postepy Hig Med Dosw (Online). 2007;61:129-50. Postepy Hig Med Dosw (Online). 2007. PMID: 17410054 Review.
Cited by
-
Immunomodulatory effects of recombinant lactoferrin during MRSA infection.Int Immunopharmacol. 2014 May;20(1):157-63. doi: 10.1016/j.intimp.2014.02.029. Epub 2014 Mar 6. Int Immunopharmacol. 2014. PMID: 24613206 Free PMC article.
-
Oral recombinant human or mouse lactoferrin reduces Mycobacterium tuberculosis TDM induced granulomatous lung pathology.Biochem Cell Biol. 2017 Feb;95(1):148-154. doi: 10.1139/bcb-2016-0061. Epub 2016 Jul 27. Biochem Cell Biol. 2017. PMID: 28165282 Free PMC article.
-
Lactoferrin in a Context of Inflammation-Induced Pathology.Front Immunol. 2017 Nov 6;8:1438. doi: 10.3389/fimmu.2017.01438. eCollection 2017. Front Immunol. 2017. PMID: 29163511 Free PMC article. Review.
-
Effects of CHO-expressed recombinant lactoferrins on mouse dendritic cell presentation and function.Innate Immun. 2015 Jul;21(5):553-61. doi: 10.1177/1753425914564609. Epub 2014 Dec 23. Innate Immun. 2015. PMID: 25537452 Free PMC article.
-
Optimized lactoferrin as a highly promising treatment for intracerebral hemorrhage: Pre-clinical experience.J Cereb Blood Flow Metab. 2021 Jan;41(1):53-66. doi: 10.1177/0271678X20925667. Epub 2020 May 21. J Cereb Blood Flow Metab. 2021. PMID: 32438861 Free PMC article.
References
-
- Quinton LJ, Dale DC. and Nelson. S. (2005). Use of colony-stimulating factors for treatment of neutropenia and infectious diseases. In: The Neutrophils: New Outlook for Old Cells. Galbrilovich D, ed., 2nd edn. Imperial Collage Press, London, pp. 301–306
-
- Noel JG, Valente JF, Ogle JD, Cornelius J, Custer DA, Li BG, Alexander JW. and Ogle CK. (2002). Changes in bone marrow-derived myeloid cells from thermally injured rats reflect changes in the progenitor cell population. J Burn Care Rehabil 23:75–86 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

