Evaluation of the impact of immediate versus WHO recommendations-guided antiretroviral therapy initiation on HIV incidence: the ANRS 12249 TasP (Treatment as Prevention) trial in Hlabisa sub-district, KwaZulu-Natal, South Africa: study protocol for a cluster randomised controlled trial
- PMID: 23880306
- PMCID: PMC3750830
- DOI: 10.1186/1745-6215-14-230
Evaluation of the impact of immediate versus WHO recommendations-guided antiretroviral therapy initiation on HIV incidence: the ANRS 12249 TasP (Treatment as Prevention) trial in Hlabisa sub-district, KwaZulu-Natal, South Africa: study protocol for a cluster randomised controlled trial
Abstract
Background: Antiretroviral therapy (ART) suppresses HIV viral load in all body compartments and so limits the risk of HIV transmission. It has been suggested that ART not only contributes to preventing transmission at individual but potentially also at population level. This trial aims to evaluate the effect of ART initiated immediately after identification/diagnosis of HIV-infected individuals, regardless of CD4 count, on HIV incidence in the surrounding population. The primary outcome of the overall trial will be HIV incidence over two years. Secondary outcomes will include i) socio-behavioural outcomes (acceptability of repeat HIV counselling and testing, treatment acceptance and linkage to care, sexual partnerships and quality of life); ii) clinical outcomes (mortality and morbidity, retention into care, adherence to ART, virologic failure and acquired HIV drug resistance), iii) cost-effectiveness of the intervention. The first phase will specifically focus on the trial's secondary outcomes.
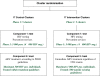
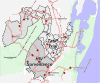
Methods/design: A cluster-randomised trial in 34 (2 × 17) clusters within a rural area of northern KwaZulu-Natal (South Africa), covering a total population of 34,000 inhabitants aged 16 years and above, of whom an estimated 27,200 would be HIV-uninfected at start of the trial. The first phase of the trial will include ten (2 × 5) clusters. Consecutive rounds of home-based HIV testing will be carried out. HIV-infected participants will be followed in dedicated trial clinics: in intervention clusters, they will be offered immediate ART initiation regardless of CD4 count and clinical stage; in control clusters they will be offered ART according to national treatment eligibility guidelines (CD4 <350 cells/μL, World Health Organisation stage 3 or 4 disease or multidrug-resistant/extensively drug-resistant tuberculosis). Following proof of acceptability and feasibility from the first phase, the trial will be rolled out to further clusters.
Discussion: We aim to provide proof-of-principle evidence regarding the effectiveness of Treatment-as-Prevention in reducing HIV incidence at the population level. Data collected from the participants at home and in the clinics will inform understanding of socio-behavioural, economic and clinical impacts of the intervention as well as feasibility and generalizability.
Trial registration: Clinicaltrials.gov: NCT01509508; South African Trial Register: DOH-27-0512-3974.
Figures
Similar articles
-
Uptake of Home-Based HIV Testing, Linkage to Care, and Community Attitudes about ART in Rural KwaZulu-Natal, South Africa: Descriptive Results from the First Phase of the ANRS 12249 TasP Cluster-Randomised Trial.PLoS Med. 2016 Aug 9;13(8):e1002107. doi: 10.1371/journal.pmed.1002107. eCollection 2016 Aug. PLoS Med. 2016. PMID: 27504637 Free PMC article. Clinical Trial.
-
Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial.Lancet HIV. 2018 Mar;5(3):e116-e125. doi: 10.1016/S2352-3018(17)30205-9. Epub 2017 Nov 30. Lancet HIV. 2018. PMID: 29199100 Clinical Trial.
-
Impact of early initiation versus national standard of care of antiretroviral therapy in Swaziland's public sector health system: study protocol for a stepped-wedge randomized trial.Trials. 2017 Aug 18;18(1):383. doi: 10.1186/s13063-017-2128-8. Trials. 2017. PMID: 28821264 Free PMC article. Clinical Trial.
-
Opportunities and Challenges in HIV Treatment as Prevention Research: Results from the ANRS 12249 Cluster-Randomized Trial and Associated Population Cohort.Curr HIV/AIDS Rep. 2020 Apr;17(2):97-108. doi: 10.1007/s11904-020-00487-1. Curr HIV/AIDS Rep. 2020. PMID: 32072468 Free PMC article. Review.
-
The REVAMP trial to evaluate HIV resistance testing in sub-Saharan Africa: a case study in clinical trial design in resource limited settings to optimize effectiveness and cost effectiveness estimates.HIV Clin Trials. 2017 Jul;18(4):149-155. doi: 10.1080/15284336.2017.1349028. Epub 2017 Jul 18. HIV Clin Trials. 2017. PMID: 28720039 Free PMC article. Review.
Cited by
-
Optimal Treatment Strategies in the Context of 'Treatment for Prevention' against HIV-1 in Resource-Poor Settings.PLoS Comput Biol. 2015 Apr 30;11(4):e1004200. doi: 10.1371/journal.pcbi.1004200. eCollection 2015 Apr. PLoS Comput Biol. 2015. PMID: 25927964 Free PMC article.
-
Changing HIV treatment eligibility under health system constraints in sub-Saharan Africa: investment needs, population health gains, and cost-effectiveness.AIDS. 2016 Sep 24;30(15):2341-50. doi: 10.1097/QAD.0000000000001190. AIDS. 2016. PMID: 27367487 Free PMC article.
-
The current status of the use of oral medication to prevent HIV transmission.Curr Opin HIV AIDS. 2015 Jul;10(4):226-32. doi: 10.1097/COH.0000000000000170. Curr Opin HIV AIDS. 2015. PMID: 26049946 Free PMC article. Review.
-
High rates of unplanned interruptions from HIV care early after antiretroviral therapy initiation in Nigeria.BMC Infect Dis. 2015 Sep 30;15:397. doi: 10.1186/s12879-015-1137-z. BMC Infect Dis. 2015. PMID: 26424505 Free PMC article.
-
Antiretroviral treatment of HIV-1 prevents transmission of HIV-1: where do we go from here?Lancet. 2013 Nov 2;382(9903):1515-24. doi: 10.1016/S0140-6736(13)61998-4. Epub 2013 Oct 23. Lancet. 2013. PMID: 24152938 Free PMC article. Review.
References
-
- UNAIDS. Report on the Global AIDS Epidemic. Geneva: UNAIDS; 2012. [ http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiolo...]. Accessed 18 January 2013.
-
- Garcia PM, Kalish LA, Pitt J, Minkoff H, Quinn TC, Burchett SK, Kornegay J, Jackson B, Moye J, Hanson C, Zorrilla C, Lew JF. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394–402. doi: 10.1056/NEJM199908053410602. - DOI - PubMed
-
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, De Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D. et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous