Regulation of Müller glial dependent neuronal regeneration in the damaged adult zebrafish retina
- PMID: 23880528
- PMCID: PMC3877724
- DOI: 10.1016/j.exer.2013.07.012
Regulation of Müller glial dependent neuronal regeneration in the damaged adult zebrafish retina
Abstract
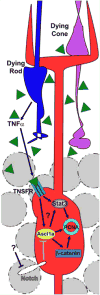
This article examines our current knowledge underlying the mechanisms involved in neuronal regeneration in the adult zebrafish retina. Zebrafish, which has the capacity to regenerate a wide variety of tissues and organs (including the fins, kidney, heart, brain, and spinal cord), has become the premier model system to study retinal regeneration due to the robustness and speed of the response and the variety of genetic tools that can be applied to study this question. It is now well documented that retinal damage induces the resident Müller glia to dedifferentiate and reenter the cell cycle to produce neuronal progenitor cells that continue to proliferate, migrate to the damaged retinal layer and differentiate into the missing neuronal cell types. Increasing our understanding of how these cellular events are regulated and occur in response to neuronal damage may provide critical information that can be applied to stimulating a regeneration response in the mammalian retina. In this review, we will focus on the genes/proteins that regulate zebrafish retinal regeneration and will attempt to critically evaluate how these factors may interact to correctly orchestrate the definitive cellular events that occur during regeneration.
Keywords: Ascl1a; Müller glia; Stat3; TNFα; dedifferentiation; neuronal progenitor cells; regeneration; zebrafish.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


References
-
- Bernardos RL, Lentz SI, Wolfe MS, Raymond PA. Notch-Delta signaling is required for spatial patterning and Muller glia differentiation in the zebrafish retina. Dev Biol. 2005;278:381–395. - PubMed
-
- Braunger BM, Ohlmann A, Koch M, Tanimoto N, Volz C, Yang Y, Bosl MR, Cvekl A, Jagle H, Seeliger MW, Tamm ER. Constitutive overexpression of Norrin activates Wnt/beta-catenin and endothelin-2 signaling to protect photoreceptors from light damage. Neurobiol Dis. 2013;50:1–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

