Peroxiredoxin-3 is overexpressed in prostate cancer and promotes cancer cell survival by protecting cells from oxidative stress
- PMID: 23880827
- PMCID: PMC3749568
- DOI: 10.1038/bjc.2013.396
Peroxiredoxin-3 is overexpressed in prostate cancer and promotes cancer cell survival by protecting cells from oxidative stress
Abstract
Objective: We have previously identified peroxiredoxin-3 (PRDX-3) as a cell-surface protein that is androgen regulated in the LNCaP prostate cancer (PCa) cell line. PRDX-3 is a member of the peroxiredoxin family that are responsible for neutralising reactive oxygen species.
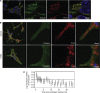
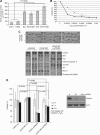
Experimental design: PRDX-3 expression was examined in tissue from 32 patients using immunohistochemistry. Subcellular distribution was determined using confocal microscopy. PRDX-3 expression was determined in antiandrogen-resistant cell lines by western blotting and quantitative RT-PCR. The pathways of PRDX-3 overexpression and knockdown on apoptosis and response to oxidative stress were investigated using protein arrays.
Results: PRDX-3 is upregulated in a number of endocrine-regulated tumours; in particular in PCa and prostatic intraepithelial neoplasia. Although the majority of PRDX-3 is localised to the mitochondria, we have confirmed that PRDX-3 at the cell membrane is androgen regulated. In antiandrogen-resistant LNCaP cell lines, PRDX-3 is upregulated at the protein but not RNA level. Resistant cells also possess an upregulation of the tricarboxylic acid (TCA) pathway and resistance to H₂O₂-induced apoptosis through a failure to activate pro-apoptotic pathways. Knockdown of PRDX-3 restored H₂O₂ sensitivity.
Conclusion: Our results suggest that PRDX-3 has an essential role in regulating oxidation-induced apoptosis in antiandrogen-resistant cells. PRDX-3 may have potential as a therapeutic target in castrate-independent PCa.
Figures


References
-
- Ambruso DR, Ellison MA, Thurman GW, Leto TL. Peroxiredoxin 6 translocates to the plasma membrane during neutrophil activation and is required for optimal NADPH oxidase activity. Biochim Biophys Acta. 2012;1823 (2:306–315. - PubMed
-
- Araki M, Nanri H, Ejima K, Murasato Y, Fujiwara T, Nakashima Y, Ikeda M. Antioxidant function of the mitochondrial protein SP-22 in the cardiovascular system. J Biol Chem. 1999;274 (4:2271–2278. - PubMed
-
- Aran M, Ferrero DS, Pagano E, Wolosiuk RA. Typical 2-Cys peroxiredoxins--modulation by covalent transformations and noncovalent interactions. FEBS J. 2009;276 (9:2478–2493. - PubMed
-
- Besada V, Diaz M, Becker M, Ramos Y, Castellanos-Serra L, Fichtner I. Proteomics of xenografted human breast cancer indicates novel targets related to tamoxifen resistance. Proteomics. 2006;6 (3:1038–1048. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

