Cell cycle regulation by long non-coding RNAs
- PMID: 23880895
- PMCID: PMC3830198
- DOI: 10.1007/s00018-013-1423-0
Cell cycle regulation by long non-coding RNAs
Abstract
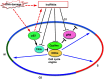
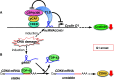
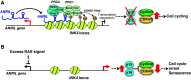
The mammalian cell cycle is precisely controlled by cyclin-dependent kinases (CDKs) and related pathways such as the RB and p53 pathways. Recent research on long non-coding RNAs (lncRNAs) indicates that many lncRNAs are involved in the regulation of critical cell cycle regulators such as the cyclins, CDKs, CDK inhibitors, pRB, and p53. These lncRNAs act as epigenetic regulators, transcription factor regulators, post-transcription regulators, and protein scaffolds. These cell cycle-regulated lncRNAs mainly control cellular levels of cell cycle regulators via various mechanisms, and may provide diversity and reliability to the general cell cycle. Interestingly, several lncRNAs are induced by DNA damage and participate in cell cycle arrest or induction of apoptosis as DNA damage responses. Therefore, deregulations of these cell cycle regulatory lncRNAs may be involved in tumorigenesis, and they are novel candidate molecular targets for cancer therapy and diagnosis.
Figures


References
-
- Nurse P. Cyclin dependent kinases and cell cycle control (nobel lecture) Chem Biochem. 2002;3:596–603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

