Abuse-related effects of µ-opioid analgesics in an assay of intracranial self-stimulation in rats: modulation by chronic morphine exposure
- PMID: 23881045
- PMCID: PMC3864000
- DOI: 10.1097/FBP.0b013e328364c0bd
Abuse-related effects of µ-opioid analgesics in an assay of intracranial self-stimulation in rats: modulation by chronic morphine exposure
Abstract
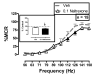
Intracranial self-stimulation (ICSS) is an operant procedure in which responding is maintained by electrical brain stimulation. Stimulation frequency can be varied rapidly to maintain a wide range of baseline response rates, and drugs' effects can be evaluated simultaneously on both low ICSS rates maintained by low stimulation frequencies and high ICSS rates maintained by high stimulation frequencies. ICSS 'facilitation' indicates drug-induced increases in low ICSS rates and is often considered an abuse-related effect, whereas ICSS 'depression' indicates decreases in high ICSS rates and may indicate abuse-limiting effects. This study examined the roles of µ-agonist efficacy and of previous µ-agonist exposure as determinants of µ-agonist effects on ICSS in rats with electrodes implanted into the medial forebrain bundle. The high-efficacy, intermediate-efficacy, and low-efficacy µ agonists methadone, fentanyl, and nalbuphine were tested during escalating regimens of morphine exposure (vehicle, 3.2, and 18 mg/kg/day). During vehicle treatment, methadone and fentanyl primarily depressed ICSS, whereas nalbuphine produced weak facilitation that was not dose dependent. Chronic morphine produced tolerance to ICSS depression and increased expression of ICSS facilitation. These results suggest that µ-agonist exposure increases the expression of abuse-related ICSS facilitation by µ agonists with a broad range of efficacies at µ receptors.
Figures





References
-
- Broekkamp CL, Van den Bogaard JH, Heijnen HJ, Rops RH, Cools AR, Van Rossum JM. Separation of inhibiting and stimulating effects of morphine on self-stimulation behaviour by intracerebral microinjections. Eur J Pharmacol. 1976;36:443–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

