The membrane transport and polyglutamation of pralatrexate: a new-generation dihydrofolate reductase inhibitor
- PMID: 23881211
- PMCID: PMC3784351
- DOI: 10.1007/s00280-013-2231-9
The membrane transport and polyglutamation of pralatrexate: a new-generation dihydrofolate reductase inhibitor
Abstract
Purpose: To characterize, directly and for the first time, the membrane transport and metabolism of pralatrexate, a new-generation dihydrofolate reductase inhibitor approved for the treatment for peripheral T-cell lymphoma.
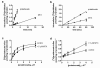
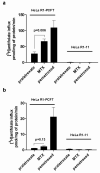
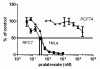
Experimental design: [(3)H]pralatrexate transport was studied in unique HeLa cell lines that express either the reduced folate carrier (RFC) or the proton-coupled folate transporter (PCFT). Metabolism to active polyglutamate derivatives was assessed by liquid chromatography. These properties were compared to those of methotrexate (MTX).
Results: The pralatrexate influx K t, mediated by RFC, the major route of folate/antifolate transport at systemic pH, was 0.52 μΜ, 1/10th the MTX influx K i. The electrochemical potential of pralatrexate within HeLa cells far exceeded the extracellular level and was greater than for MTX. In contrast, MTX transport mediated by PCFT, the mechanism of folate/antifolate absorption in the small intestine, exceeded that for pralatrexate. After a 6 h exposure of HeLa cells to 0.5 μM pralatrexate, 80 % of intracellular drug was its active polyglutamate forms, predominantly the tetraglutamate, and was suppressed when cells were loaded with natural folates. There was negligible formation of MTX polyglutamates. The difference in pralatrexate and MTX growth inhibition was far greater after transient exposures (375-fold) than continuous exposure (25-fold) to the drugs.
Conclusions: Pralatrexate's enhanced activity relative to MTX is due to its much more rapid rate of transport and polyglutamation, the former less important when the carrier is saturated. The low affinity of pralatrexate for PCFT predicts a lower level of enterohepatic circulation and increased fecal excretion of the drug relative to MTX.
Figures


Similar articles
-
The major facilitative folate transporters solute carrier 19A1 and solute carrier 46A1: biology and role in antifolate chemotherapy of cancer.Drug Metab Dispos. 2014 Apr;42(4):632-49. doi: 10.1124/dmd.113.055723. Epub 2014 Jan 6. Drug Metab Dispos. 2014. PMID: 24396145 Free PMC article. Review.
-
5-amino-4-imidazolecarboxamide riboside potentiates both transport of reduced folates and antifolates by the human reduced folate carrier and their subsequent metabolism.Cancer Res. 2006 Apr 1;66(7):3836-44. doi: 10.1158/0008-5472.CAN-05-3226. Cancer Res. 2006. PMID: 16585211
-
Concentrative Transport of Antifolates Mediated by the Proton-Coupled Folate Transporter (SLC46A1); Augmentation by a HEPES Buffer.Mol Pharmacol. 2018 Mar;93(3):208-215. doi: 10.1124/mol.117.110445. Epub 2018 Jan 11. Mol Pharmacol. 2018. PMID: 29326243 Free PMC article.
-
Biology of the major facilitative folate transporters SLC19A1 and SLC46A1.Curr Top Membr. 2014;73:175-204. doi: 10.1016/B978-0-12-800223-0.00004-9. Curr Top Membr. 2014. PMID: 24745983 Free PMC article. Review.
-
The mechanism of transport of the multitargeted antifolate (MTA) and its cross-resistance pattern in cells with markedly impaired transport of methotrexate.Clin Cancer Res. 2000 Sep;6(9):3687-95. Clin Cancer Res. 2000. PMID: 10999762
Cited by
-
Leucovorin rescue allows effective high-dose pralatrexate treatment and an increase in therapeutic index in mesothelioma xenografts.Cancer Chemother Pharmacol. 2014 Nov;74(5):1029-32. doi: 10.1007/s00280-014-2580-z. Epub 2014 Sep 9. Cancer Chemother Pharmacol. 2014. PMID: 25205429 Free PMC article.
-
The impact of 5-formyltetrahydrofolate on the anti-tumor activity of pralatrexate, as compared to methotrexate, in HeLa cells in vitro.Cancer Chemother Pharmacol. 2014 May;73(5):1055-62. doi: 10.1007/s00280-014-2441-9. Epub 2014 Mar 29. Cancer Chemother Pharmacol. 2014. PMID: 24682532 Free PMC article.
-
Methylthioadenosine phosphorylase (MTAP)-deficient T-cell ALL xenografts are sensitive to pralatrexate and 6-thioguanine alone and in combination.Cancer Chemother Pharmacol. 2015 Jun;75(6):1247-52. doi: 10.1007/s00280-015-2747-2. Epub 2015 Apr 28. Cancer Chemother Pharmacol. 2015. PMID: 25917288 Free PMC article.
-
Harnessing the immune system in the treatment of cutaneous T cell lymphomas.Front Oncol. 2023 Jan 12;12:1071171. doi: 10.3389/fonc.2022.1071171. eCollection 2022. Front Oncol. 2023. PMID: 36713518 Free PMC article.
-
The major facilitative folate transporters solute carrier 19A1 and solute carrier 46A1: biology and role in antifolate chemotherapy of cancer.Drug Metab Dispos. 2014 Apr;42(4):632-49. doi: 10.1124/dmd.113.055723. Epub 2014 Jan 6. Drug Metab Dispos. 2014. PMID: 24396145 Free PMC article. Review.
References
-
- Bertino JR. Ode to methotrexate. J Clin Oncol. 1993;11:5–14. - PubMed
-
- Farber S, Diamond LK, Mercer RD, Sylvester RF, Wolff VA. Temporary remission in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl glutamic acid (aminopterin) N Engl J Med. 1948;238:787–793. - PubMed
-
- Osborn MJ, Huennekens FM. Enzymatic reduction of dihydrofolic acid. J Biol Chem. 1958;233:969–974. - PubMed
-
- Zhao R, Goldman ID. Resistance to antifolates. Oncogene. 2003;22:7431–7457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

