Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis
- PMID: 23881354
- PMCID: PMC3859808
- DOI: 10.1038/mi.2013.52
Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis
Abstract
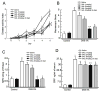
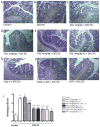
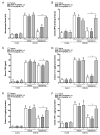
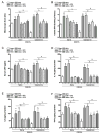
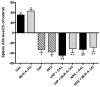
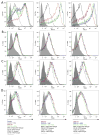
The cholinergic anti-inflammatory pathway is an efferent vagus nerve-based mechanism that regulates immune responses and cytokine production through α7 nicotinic acetylcholine receptor (α7nAChR) signaling. Decreased efferent vagus nerve activity is observed in inflammatory bowel disease. We determined whether central activation of this pathway alters inflammation in mice with colitis and the mediating role of a vagus nerve-to-spleen circuit and α7nAChR signaling. Two experimental models of colitis were used in C57BL/6 mice. Central cholinergic activation induced by the acetylcholinesterase inhibitor galantamine or a muscarinic acetylcholine receptor agonist treatments resulted in reduced mucosal inflammation associated with decreased major histocompatibility complex II level and pro-inflammatory cytokine secretion by splenic CD11c⁺ cells mediated by α7nAChR signaling. The cholinergic anti-inflammatory efficacy was abolished in mice with vagotomy, splenic neurectomy, or splenectomy. In conclusion, central cholinergic activation of a vagus nerve-to-spleen circuit controls intestinal inflammation and this regulation can be explored to develop novel therapeutic strategies.
Figures


References
-
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. e42. quiz e30. - PubMed
-
- Perrier C, Rutgeerts P. Cytokine blockade in inflammatory bowel diseases. Immunotherapy. 2011;3:1341–1352. - PubMed
-
- Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–1130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

