Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials
- PMID: 23881988
- PMCID: PMC3780632
- DOI: 10.1634/theoncologist.2013-0107
Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials
Abstract
Purpose: his analysis pooled individual patient data from randomized controlled trials (RCTs) to more thoroughly examine clinical outcomes when adding bevacizumab to chemotherapy for patients with metastatic colorectal cancer (mCRC).
Patients and methods: Patient data were pooled from the first-line AVF2107, NO16966, ARTIST, AVF0780, AVF2192, and AGITG MAX RCTs and the second-line E3200 RCT. All analyses were based on the intent-to-treat population. To assess differences in time-to-event variables by treatment (chemotherapy with or without placebo vs. chemotherapy plus bevacizumab), stratified random-effects (overall) and fixed-effects (subgroup comparisons) models were used to estimate pooled hazard ratios (HRs) and 95% confidence intervals (CIs).
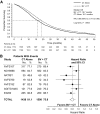
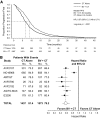
Results: The analysis population comprised 3,763 patients (1,773 chemotherapy with or without placebo; 1,990 chemotherapy plus bevacizumab). The addition of bevacizumab to chemotherapy was associated with statistically significant increases in overall survival (OS; HR, 0.80; 95% CI, 0.71-0.90) and progression-free survival (PFS; HR, 0.57; 95% CI, 0.46-0.71). The effects on OS and PFS across subgroups defined by chemotherapy backbone (oxaliplatin-based, irinotecan-based), extent of disease (liver metastases only, extensive disease), age (<65, ≥65 years), Eastern Cooperative Oncology Group performance status (0, ≥1), and KRAS status (wild-type, mutant) were consistent with the overall analysis. Incidence rates of grade ≥3 hypertension, proteinuria, bleeding, wound-healing complications, gastrointestinal perforations, and thromboembolic events were increased with bevacizumab treatment.
Conclusion: The use of bevacizumab with chemotherapy resulted in statistically significant increases in OS and PFS for patients with mCRC. The PFS benefit extended across the clinically relevant subgroups examined. The observed safety profile of bevacizumab was consistent with that reported in individual trials.
Keywords: Angiogenesis inhibitors; Antibodies, monoclonal, humanized; Bevacizumab; Colorectal neoplasms.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. - PubMed
-
- Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: Results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. - PubMed
-
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. - PubMed
-
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. - PubMed
-
- Tebbutt NC, Wilson K, Gebski VJ, et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: Results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28:3191–3198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

