Optic ataxia as a model to investigate the role of the posterior parietal cortex in visually guided action: evidence from studies of patient M.H
- PMID: 23882200
- PMCID: PMC3712225
- DOI: 10.3389/fnhum.2013.00336
Optic ataxia as a model to investigate the role of the posterior parietal cortex in visually guided action: evidence from studies of patient M.H
Abstract
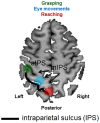
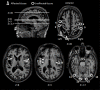
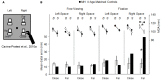
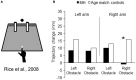
Optic ataxia is a neuropsychological disorder that affects the ability to interact with objects presented in the visual modality following either unilateral or bilateral lesions of the posterior parietal cortex (PPC). Patients with optic ataxia fail to reach accurately for objects, particularly when they are presented in peripheral vision. The present review will focus on a series of experiments performed on patient M.H. Following a lesion restricted largely to the left PPC, he developed mis-reaching behavior when using his contralesional right arm for movements directed toward the contralesional (right) visual half-field. Given the clear-cut specificity of this patient's deficit, whereby reaching actions are essentially spared when executed toward his ipsilateral space or when using his left arm, M.H. provides a valuable "experiment of nature" for investigating the role of the PPC in performing different visually guided actions. In order to address this, we used kinematic measurement techniques to investigate M.H.'s reaching and grasping behavior in various tasks. Our experiments support the idea that optic ataxia is highly function-specific: it affects a specific sub-category of visually guided actions (reaching but not grasping), regardless of their specific end goal (both reaching toward an object and reaching to avoid an obstacle); and finally, is independent of the limb used to perform the action (whether the arm or the leg). Critically, these results are congruent with recent functional MRI experiments in neurologically intact subjects which suggest that the PPC is organized in a function-specific, rather than effector-specific, manner with different sub-portions of its mantle devoted to guiding actions according to their specific end-goal (reaching, grasping, or looking), rather than according to the effector used to perform them (leg, arm, hand, or eyes).
Keywords: arm; grasping; hand; leg; optic ataxia; posterior parietal cortex; reaching.
Figures
References
-
- Andersen R. A. (1987). Neurons of area 7 activated by both visual stimuli and oculomotor behavior, in The Handbook of Physiology. Section I: The Nervous System Volume V. Higher Functions of the Brain Part 2, eds Plum M. F., Mountcastle V. B., Geiger S. R. (Bethesda, MD: American Physiological Society; ), 483–518
LinkOut - more resources
Full Text Sources
Other Literature Sources

