Membrane potential and cancer progression
- PMID: 23882223
- PMCID: PMC3713347
- DOI: 10.3389/fphys.2013.00185
Membrane potential and cancer progression
Abstract
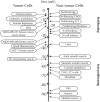
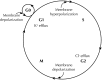
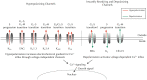
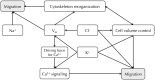
Membrane potential (Vm ), the voltage across the plasma membrane, arises because of the presence of different ion channels/transporters with specific ion selectivity and permeability. Vm is a key biophysical signal in non-excitable cells, modulating important cellular activities, such as proliferation and differentiation. Therefore, the multiplicities of various ion channels/transporters expressed on different cells are finely tuned in order to regulate the Vm . It is well-established that cancer cells possess distinct bioelectrical properties. Notably, electrophysiological analyses in many cancer cell types have revealed a depolarized Vm that favors cell proliferation. Ion channels/transporters control cell volume and migration, and emerging data also suggest that the level of Vm has functional roles in cancer cell migration. In addition, hyperpolarization is necessary for stem cell differentiation. For example, both osteogenesis and adipogenesis are hindered in human mesenchymal stem cells (hMSCs) under depolarizing conditions. Therefore, in the context of cancer, membrane depolarization might be important for the emergence and maintenance of cancer stem cells (CSCs), giving rise to sustained tumor growth. This review aims to provide a broad understanding of the Vm as a bioelectrical signal in cancer cells by examining several key types of ion channels that contribute to its regulation. The mechanisms by which Vm regulates cancer cell proliferation, migration, and differentiation will be discussed. In the long term, Vm might be a valuable clinical marker for tumor detection with prognostic value, and could even be artificially modified in order to inhibit tumor growth and metastasis.
Keywords: cancer; cell cycle; differentiation; ion channel; membrane potential; migration; proliferation; stem cell.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

