Metabolic engineering of raffinose-family oligosaccharides in the phloem reveals alterations in carbon partitioning and enhances resistance to green peach aphid
- PMID: 23882277
- PMCID: PMC3715723
- DOI: 10.3389/fpls.2013.00263
Metabolic engineering of raffinose-family oligosaccharides in the phloem reveals alterations in carbon partitioning and enhances resistance to green peach aphid
Abstract
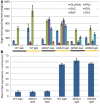
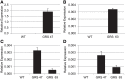
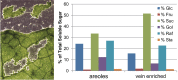
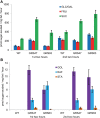
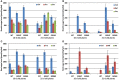
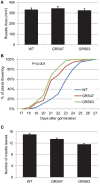
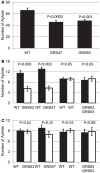
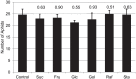
Many plants employ energized loading strategies to accumulate osmotically-active solutes into the phloem of source organs to accentuate the hydrostatic pressure gradients that drive the flow of water, nutrients and signals from source to sinks. Proton-coupled symport of sugars from the apoplasm into the phloem symplasm is the best studied phloem-loading mechanism. As an alternative, numerous species use a polymer trapping mechanism to load through symplasm: sucrose enters the phloem through specialized plasmodesmata and is converted to raffinose-family oligosaccharides (RFOs) which accumulate because of their larger size. In this study, metabolic engineering was used to generate RFOs at the inception of the translocation stream of Arabidopsis thaliana, which loads from the apoplasm and transports predominantly sucrose, and the fate of the sugars throughout the plant determined. Three genes, GALACTINOL SYNTHASE, RAFFINOSE SYNTHASE and STACHYOSE SYNTHASE, were expressed from promoters specific to the companion cells of minor veins. Two transgenic lines homozygous for all three genes (GRS63 and GRS47) were selected for further analysis. Three-week-old plants of both lines had RFO levels approaching 50% of total soluble sugar. RFOs were also identified in exudates from excised leaves of transgenic plants whereas levels were negligible in exudates from wild type (WT) leaves. Differences in starch accumulation between WT and GRS63 and GRS47 lines were not observed. Similarly, there were no differences in vegetative growth between WT and engineered plants, but the latter flowered slightly earlier. Finally, since the sugar composition of the translocation stream appeared altered, we tested for an impact on green peach aphid (Myzus persicae Sulzer) feeding. When given a choice between WT and transgenic plants, green peach aphids preferred settling on the WT plants. Furthermore, green peach aphid fecundity was lower on the transgenic plants compared to the WT plants. When added to an artificial diet, RFOs did not have a negative effect on aphid fecundity, suggesting that although aphid resistance in the transgenic plants is enhanced, it is not due to direct toxicity of RFO toward the insect.
Keywords: green peach aphid; metabolic engineering; phloem transport; raffinose family oligosaccharides; sugar transport.
Figures

References
-
- Bailly C., Audigier C., Ladonne F., Wagner M. H., Coste F., Corbineau F., et al. (2001). Changes in oligosaccharide content and antioxidant enzyme activities in developing bean seeds as related to acquisition of drying tolerance and seed quality. J. Exp. Bot. 52, 701–708 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

