Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged ≥18 years
- PMID: 23883186
- PMCID: PMC3750613
- DOI: 10.1186/1471-2334-13-343
Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged ≥18 years
Abstract
Background: Two antigenically distinct influenza B lineages have co-circulated since the 1980s, yet inactivated trivalent influenza vaccines (TIVs) include strains of influenza A/H1N1, A/H3N2, and only one influenza B from either the Victoria or Yamagata lineage. This means that exposure to B-lineage viruses mismatched to the TIV is frequent, reducing vaccine protection. Formulations including both influenza B lineages could improve protection against circulating influenza B viruses. We assessed a candidate inactivated quadrivalent influenza vaccine (QIV) containing both B lineages versus TIV in adults in stable health.
Methods: A total of 4659 adults were randomized 5:5:5:5:3 to receive one dose of QIV (one of three lots) or a TIV containing either a B/Victoria or B/Yamagata strain. Hemagglutination-inhibition assays were performed pre-vaccination and 21-days after vaccination. Lot-to-lot consistency of QIV was assessed based on geometric mean titers (GMT). For QIV versus TIV, non-inferiority against the three shared strains was demonstrated if the 95% confidence interval (CI) upper limit for the GMT ratio was ≤1.5 and for the seroconversion difference was ≤10.0%; superiority of QIV versus TIV for the alternate B lineage was demonstrated if the 95% CI lower limit for the GMT ratio was > 1.0 and for the seroconversion difference was > 0%. Reactogenicity and safety profile of each vaccine were assessed. Clinicaltrials.gov: NCT01204671.
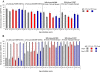
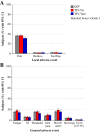
Results: Consistent immunogenicity was demonstrated for the three QIV lots. QIV was non-inferior to TIV for the shared vaccine strains, and was superior for the added alternate-lineage B strains. QIV elicited robust immune responses against all four vaccine strains; the seroconversion rates were 77.5% (A/H1N1), 71.5% (A/H3N2), 58.1% (B/Victoria), and 61.7% (B/Yamagata). The reactogenicity and safety profile of QIV was consistent with TIV.
Conclusions: QIV provided superior immunogenicity for the additional B strain compared with TIV, without interfering with antibody responses to the three shared antigens. The additional antigen did not appear to alter the safety profile of QIV compared with TIV. This suggests that the candidate QIV is a viable alternative to TIV for use in adults, and could potentially improve protection against influenza B.
Trial registration: Clinical Trials.gov: NCT01204671/114269.
Figures
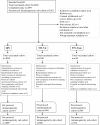

References
-
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS. et al. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62. - PubMed
-
- Ampofo WK, Baylor N, Cobey S, Cox NJ, Daves S, Edwards S, Ferguson N, Grohmann G, Hay A, Katz J. et al. Improving influenza vaccine virus selection: report of a WHO informal consultation held at WHO headquarters, Geneva, Switzerland, 14–16 June 2010. Influenza Other Respi Viruses. 2012;6(2):142–152. e141-145. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

