Human platelets generate phospholipid-esterified prostaglandins via cyclooxygenase-1 that are inhibited by low dose aspirin supplementation
- PMID: 23883581
- PMCID: PMC3793613
- DOI: 10.1194/jlr.M041533
Human platelets generate phospholipid-esterified prostaglandins via cyclooxygenase-1 that are inhibited by low dose aspirin supplementation
Abstract
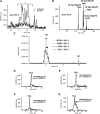
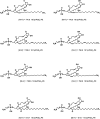
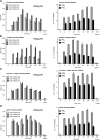
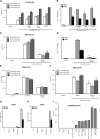
Oxidized phospholipids (oxPLs) generated nonenzymatically display pleiotropic biological actions in inflammation. Their generation by cellular cyclooxygenases (COXs) is currently unknown. To determine whether platelets generate prostaglandin (PG)-containing oxPLs, then characterize their structures and mechanisms of formation, we applied precursor scanning-tandem mass spectrometry to lipid extracts of agonist-activated human platelets. Thrombin, collagen, or ionophore activation stimulated generation of families of PGs comprising PGE₂ and D₂ attached to four phosphatidylethanolamine (PE) phospholipids (16:0p/, 18:1p/, 18:0p/, and 18:0a/). They formed within 2 to 5 min of activation in a calcium, phospholipase C, p38 MAP kinases, MEK1, cPLA₂, and src tyrosine kinase-dependent manner (28.1 ± 2.3 pg/2 × 10⁸ platelets). Unlike free PGs, they remained cell associated, suggesting an autocrine mode of action. Their generation was inhibited by in vivo aspirin supplementation (75 mg/day) or in vitro COX-1 blockade. Inhibitors of fatty acyl reesterification blocked generation significantly, while purified COX-1 was unable to directly oxidize PE in vitro. This indicates that they form in platelets via rapid esterification of COX-1 derived PGE₂/D₂ into PE. In summary, COX-1 in human platelets acutely mediates membrane phospholipid oxidation via formation of PG-esterified PLs in response to pathophysiological agonists.
Keywords: Oxidized phospholipids; PGE2/D2-PEs; atherosclerosis.
Figures


References
-
- Kozak K. R., Rowlinson S. W., Marnett L. J. 2000. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J. Biol. Chem. 275: 33744–33749 - PubMed
-
- Kozak K. R., Crews B. C., Morrow J. D., Wang L. H., Ma Y. H., Weinander R., Jakobsson P. J., Marnett L. J. 2002. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J. Biol. Chem. 277: 44877–44885 - PubMed
-
- Hammond V. J., Morgan A. H., Lauder S., Thomas C. P., Brown S., Freeman B. A., Lloyd C. M., Davies J., Bush A., Levonen A. L., et al. 2012. Novel keto-phospholipids are generated by monocytes and macrophages, detected in cystic fibrosis, and activate peroxisome proliferator-activated receptor-gamma. J. Biol. Chem. 287: 41651–41666 - PMC - PubMed
-
- Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Febbraio M., Hajjar D. P., et al. 2002. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 277: 38517–38523 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

