Feasibility of fully automated closed-loop glucose control using continuous subcutaneous glucose measurements in critical illness: a randomized controlled trial
- PMID: 23883613
- PMCID: PMC4056260
- DOI: 10.1186/cc12838
Feasibility of fully automated closed-loop glucose control using continuous subcutaneous glucose measurements in critical illness: a randomized controlled trial
Abstract
Introduction: Closed-loop (CL) systems modulate insulin delivery according to glucose levels without nurse input. In a prospective randomized controlled trial, we evaluated the feasibility of an automated closed-loop approach based on subcutaneous glucose measurements in comparison with a local sliding-scale insulin-therapy protocol.
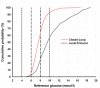
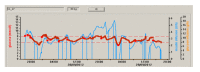
Methods: Twenty-four critically ill adults (predominantly trauma and neuroscience patients) with hyperglycemia (glucose, ≥10 mM) or already receiving insulin therapy, were randomized to receive either fully automated closed-loop therapy (model predictive control algorithm directing insulin and 20% dextrose infusion based on FreeStyle Navigator continuous subcutaneous glucose values, n = 12) or a local protocol (n = 12) with intravenous sliding-scale insulin, over a 48-hour period. The primary end point was percentage of time when arterial blood glucose was between 6.0 and 8.0 mM.
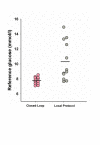
Results: The time when glucose was in the target range was significantly increased during closed-loop therapy (54.3% (44.1 to 72.8) versus 18.5% (0.1 to 39.9), P = 0.001; median (interquartile range)), and so was time in wider targets, 5.6 to 10.0 mM and 4.0 to 10.0 mM (P ≤ 0.002), reflecting a reduced glucose exposure >8 and >10 mM (P ≤ 0.002). Mean glucose was significantly lower during CL (7.8 (7.4 to 8.2) versus 9.1 (8.3 to 13.0] mM; P = 0.001) without hypoglycemia (<4 mM) during either therapy.
Conclusions: Fully automated closed-loop control based on subcutaneous glucose measurements is feasible and may provide efficacious and hypoglycemia-free glucose control in critically ill adults.
Trial registration: ClinicalTrials.gov Identifier, NCT01440842.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

