Daily electronic self-monitoring of subjective and objective symptoms in bipolar disorder--the MONARCA trial protocol (MONitoring, treAtment and pRediCtion of bipolAr disorder episodes): a randomised controlled single-blind trial
- PMID: 23883891
- PMCID: PMC3731717
- DOI: 10.1136/bmjopen-2013-003353
Daily electronic self-monitoring of subjective and objective symptoms in bipolar disorder--the MONARCA trial protocol (MONitoring, treAtment and pRediCtion of bipolAr disorder episodes): a randomised controlled single-blind trial
Abstract
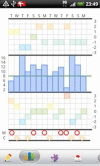
Introduction: Electronic self-monitoring of affective symptoms using cell phones is suggested as a practical and inexpensive way to monitor illness activity and identify early signs of affective symptoms. It has never been tested in a randomised clinical trial whether electronic self-monitoring improves outcomes in bipolar disorder. We are conducting a trial testing the effect of using a Smartphone for self-monitoring in bipolar disorder.
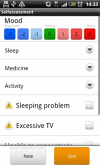
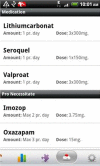
Methods: We developed the MONARCA application for Android-based Smartphones, allowing patients suffering from bipolar disorder to do daily self-monitoring-including an interactive feedback loop between patients and clinicians through a web-based interface. The effect of the application was tested in a parallel-group, single-blind randomised controlled trial so far including 78 patients suffering from bipolar disorder in the age group 18-60 years who were given the use of a Smartphone with the MONARCA application (intervention group) or to the use of a cell phone without the application (placebo group) during a 6-month study period. The study was carried out from September 2011. The outcomes were changes in affective symptoms (primary), social functioning, perceived stress, self-rated depressive and manic symptoms, quality of life, adherence to medication, stress and cognitive functioning (secondary and tertiary).
Analysis: Recruitment is ongoing.
Ethics: Ethical permission has been obtained.
Dissemination: Positive, neutral and negative findings of the study will be published.
Registration details: The trial is approved by the Regional Ethics Committee in The Capital Region of Denmark (H-2-2011-056) and The Danish Data Protection Agency (2013-41-1710). The trial is registered at ClinicalTrials.gov as NCT01446406.
Keywords: Medical Education & Training; Mental Health.
Figures
References
-
- Pini S, de Queiroz V, Pagnin D, et al. Prevalence and burden of bipolar disorders in European countries. Eur Neuropsychopharmacol 2005;15:425–34 - PubMed
-
- Sherazi R, McKeon P, McDonough M, et al. What's new? The clinical epidemiology of bipolar I disorder. Harv Rev Psychiatry 2006;14:273–84 - PubMed
-
- Angst J, Sellaro R. Historical perspectives and natural history of bipolar disorder. Biol Psychiatry 2000;48:445–57 - PubMed
-
- Kessing LV, Andersen PK, Mortensen PB, et al. Recurrence in affective disorder. I. Case register study. Br J Psychiatry 1998;172:23–8 - PubMed
-
- Kessing LV, Hansen MG, Andersen PK, et al. The predictive effect of episodes on the risk of recurrence in depressive and bipolar disorders—a life-long perspective. Acta Psychiatr Scand 2004;109:339–44 - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical