Developability studies before initiation of process development: improving manufacturability of monoclonal antibodies
- PMID: 23883920
- PMCID: PMC3851230
- DOI: 10.4161/mabs.25269
Developability studies before initiation of process development: improving manufacturability of monoclonal antibodies
Abstract
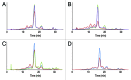
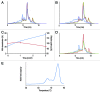
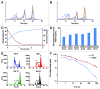
Monoclonal antibodies constitute a robust class of therapeutic proteins. Their stability, resistance to stress conditions and high solubility have allowed the successful development and commercialization of over 40 antibody-based drugs. Although mAbs enjoy a relatively high probability of success compared with other therapeutic proteins, examples of projects that are suspended due to the instability of the molecule are not uncommon. Developability assessment studies have therefore been devised to identify early during process development problems associated with stability, solubility that is insufficient to meet expected dosing or sensitivity to stress. This set of experiments includes short-term stability studies at 2-8 þC, 25 þC and 40 þC, freeze-thaw studies, limited forced degradation studies and determination of the viscosity of high concentration samples. We present here three case studies reflecting three typical outcomes: (1) no major or unexpected degradation is found and the study results are used to inform early identification of degradation pathways and potential critical quality attributes within the Quality by Design framework defined by US Food and Drug Administration guidance documents; (2) identification of specific degradation pathway(s) that do not affect potency of the molecule, with subsequent definition of proper process control and formulation strategies; and (3) identification of degradation that affects potency, resulting in program termination and reallocation of resources.
Keywords: CQA; QbD; analytics; developability; development; discovery; forced degradation; process control; stability.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
