Applications of nanotechnology for immunology
- PMID: 23883969
- PMCID: PMC7097370
- DOI: 10.1038/nri3488
Applications of nanotechnology for immunology
Erratum in
- Nat Rev Immunol. 2013 Sep;13(9):701
Abstract
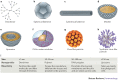
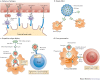
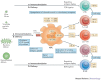
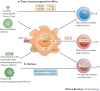
Nanotechnology uses the unique properties of objects that function as a unit within the overall size range of 1-1,000 nanometres. The engineering of nanostructure materials, including nanoparticles, nanoemulsions or nanotubules, holds great promise for the development of new immunomodulatory agents, as such nanostructures can be used to more effectively manipulate or deliver immunologically active components to target sites. Successful applications of nanotechnology in the field of immunology will enable new generations of vaccines, adjuvants and immunomodulatory drugs that aim to improve clinical outcomes in response to a range of infectious and non-infectious diseases.
Conflict of interest statement
J.R.B.J. holds an ownership stake in NanoBio Corporation, USA, and is the inventor of technologies that the University of Michigan has licensed to NanoBio Corporation.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

