Inhibitory effects of belatacept on allospecific regulatory T-cell generation in humans
- PMID: 23883971
- PMCID: PMC3800494
- DOI: 10.1097/TP.0b013e31829f1607
Inhibitory effects of belatacept on allospecific regulatory T-cell generation in humans
Abstract
Background: It is unclear if new costimulatory blockade agents, such as the cytotoxic T lymphocyte-associated antigen 4-Ig molecule belatacept (BEL), promote or inhibit the potential for immunologic tolerance in transplantation. We therefore tested the in vitro effects of BEL on human regulatory T cells (Tregs) in mixed lymphocyte reactions (MLR) alone and in combination with maintenance agents used in transplant recipients.
Methods: BEL, mycophenolic acid (MPA), and sirolimus, either alone or in combination, were added to healthy volunteer Treg-MLR, testing (a) H-TdR incorporation for inhibition of lymphoproliferation and (b) flow cytometry to analyze for newly generated CD4+ CD25(high) FOXP3+ Tregs in carboxyfluorescein succinimidyl ester-labeled MLR responders. In addition, the modulatory effects of putative Tregs generated in the presence of these drugs were also tested using the lymphoproliferation and flow cytometric assays.
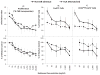
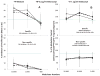
Results: In comparison with medium controls, BEL dose-dependently inhibited both lymphoproliferation and Treg generation in human leukocyte antigen DR matched and mismatched MLRs either alone or in combination with MPA or sirolimus. However, MPA alone inhibited lymphoproliferation but significantly enhanced Treg generation at subtherapeutic concentrations (P<0.01). In addition, purified CD4+ CD127- cells generated in MLR in the presence of MPA and added as third component modulators in fresh MLRs significantly enhanced newly developed Tregs in the proliferating responder cells compared with those generated with BEL or medium controls.
Conclusions: BEL alone and in combination with agents used in transplant recipients inhibits the in vitro generation of human Tregs. BEL might therefore be a less optimal agent for tolerance induction in human organ transplantation.
Figures



References
-
- Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5 (3):443. - PubMed
-
- Durrbach A, Pestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study) Am J Transplant. 2010;10 (3):547. - PubMed
-
- Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10 (3):535. - PubMed
-
- Szabo G, Gavala C, Mandrekar P. Tacrolimus and cyclosporine A inhibit allostimulatory capacity and cytokine production of human myeloid dendritic cells. Journal of Investigative Medicine. 2001;49 (5):442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

