Activation of NOD receptors by Neisseria gonorrhoeae modulates the innate immune response
- PMID: 23884094
- PMCID: PMC3880408
- DOI: 10.1177/1753425913493453
Activation of NOD receptors by Neisseria gonorrhoeae modulates the innate immune response
Abstract
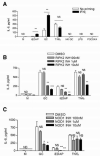
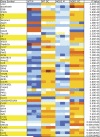
NOD1 and NOD2 are members of the NOD-like receptor family of cytosolic pattern recognition receptors that recognize specific fragments of the bacterial cell wall component peptidoglycan. Neisseria species are unique amongst Gram-negative bacteria in that they turn over large amounts of peptidoglycan during growth. We examined the ability of NOD1 and NOD2 to recognize Neisseria gonorrhoeae, and determined the role of NOD-dependent signaling in regulating the immune response to gonococcal infection. Gonococci, as well as conditioned medium from mid-logarithmic phase grown bacteria, were capable of activating both human NOD1 and NOD2, as well as mouse NOD2, leading to the activation of the transcription factor NF-κB and polyubiquitination of the adaptor receptor-interacting serine-threonine kinase 2. We identified a number of cytokines and chemokines that were differentially expressed in wild type versus NOD2-deficient macrophages in response to gonococcal infection. Moreover, NOD2 signaling up-regulated complement pathway components and cytosolic nucleic acid sensors, suggesting a broad impact of NOD activation on innate immunity. Thus, NOD1 and NOD2 are important intracellular regulators of the immune response to infection with N. gonorrhoeae. Given the intracellular lifestyle of this pathogen, we believe these cytosolic receptors may provide a key innate immune defense mechanism for the host during gonococcal infection.
Keywords: Innate immunity; NOD-like receptors; Neisseria gonorrhoeae.
Figures
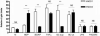
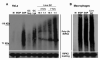


Similar articles
-
Nod2 and Rip2 contribute to innate immune responses in mouse neutrophils.Immunology. 2014 Oct;143(2):269-76. doi: 10.1111/imm.12307. Immunology. 2014. PMID: 24766550 Free PMC article.
-
Nod1 and Nod2 induce CCL5/RANTES through the NF-kappaB pathway.Eur J Immunol. 2007 Sep;37(9):2499-508. doi: 10.1002/eji.200737069. Eur J Immunol. 2007. PMID: 17705131
-
A Single Dual-Function Enzyme Controls the Production of Inflammatory NOD Agonist Peptidoglycan Fragments by Neisseria gonorrhoeae.mBio. 2017 Oct 17;8(5):e01464-17. doi: 10.1128/mBio.01464-17. mBio. 2017. PMID: 29042497 Free PMC article.
-
The role of NOD1 and NOD2 in host defense against chlamydial infection.FEMS Microbiol Lett. 2016 Sep;363(17):fnw170. doi: 10.1093/femsle/fnw170. Epub 2016 Jul 14. FEMS Microbiol Lett. 2016. PMID: 27421958 Review.
-
NOD1 and NOD2 in inflammatory and infectious diseases.Immunol Rev. 2020 Sep;297(1):139-161. doi: 10.1111/imr.12902. Epub 2020 Jul 17. Immunol Rev. 2020. PMID: 32677123 Free PMC article. Review.
Cited by
-
Neisseria gonorrhoeae host adaptation and pathogenesis.Nat Rev Microbiol. 2018 Apr;16(4):226-240. doi: 10.1038/nrmicro.2017.169. Epub 2018 Feb 12. Nat Rev Microbiol. 2018. PMID: 29430011 Free PMC article. Review.
-
Lytic transglycosylases: concinnity in concision of the bacterial cell wall.Crit Rev Biochem Mol Biol. 2017 Oct;52(5):503-542. doi: 10.1080/10409238.2017.1337705. Epub 2017 Jun 23. Crit Rev Biochem Mol Biol. 2017. PMID: 28644060 Free PMC article. Review.
-
Synthesis and validation of click-modified NOD1/2 agonists.Innate Immun. 2023 Nov;29(8):186-200. doi: 10.1177/17534259231207198. Epub 2023 Oct 13. Innate Immun. 2023. PMID: 37828863 Free PMC article.
-
Mechanisms of host manipulation by Neisseria gonorrhoeae.Front Microbiol. 2023 Feb 3;14:1119834. doi: 10.3389/fmicb.2023.1119834. eCollection 2023. Front Microbiol. 2023. PMID: 36819065 Free PMC article. Review.
-
Anti-Virulence Therapeutic Approaches for Neisseria gonorrhoeae.Antibiotics (Basel). 2021 Jan 21;10(2):103. doi: 10.3390/antibiotics10020103. Antibiotics (Basel). 2021. PMID: 33494538 Free PMC article. Review.
References
-
- CDC . Sexually Transmitted Disease Surveillance, 2009. U.S. Department of Health and Human Services; Atlanta: 2010.
-
- Hook EW, Handsfield HH. Gonococcal infections in the adult. In: Holmes KK, Sparling PF, Mardh PA, et al., editors. Sex Transm Dis. McGraw-Hill Companies, Inc; New York: 1999. pp. 451–66.
-
- Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868–73. - PubMed
-
- Levine WC, Pope V, Bhoomkar A, et al. Increase in endocervical CD4 lymphocytes among women with nonulcerative sexually transmitted diseases. The Journal of infectious diseases. 1998;177:167–74. - PubMed
-
- Chen A, Boulton IC, Pongoski J, Cochrane A, Gray-Owen SD. Induction of HIV-1 long terminal repeat-mediated transcription by Neisseria gonorrhoeae. Aids. 2003;17:625–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

