High-speed panoramic light-sheet microscopy reveals global endodermal cell dynamics
- PMID: 23884240
- PMCID: PMC3731668
- DOI: 10.1038/ncomms3207
High-speed panoramic light-sheet microscopy reveals global endodermal cell dynamics
Abstract
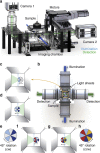
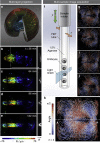
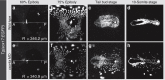
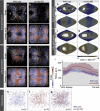
The ever-increasing speed and resolution of modern microscopes make the storage and post-processing of images challenging and prevent thorough statistical analyses in developmental biology. Here, instead of deploying massive storage and computing power, we exploit the spherical geometry of zebrafish embryos by computing a radial maximum intensity projection in real time with a 240-fold reduction in data rate. In our four-lens selective plane illumination microscope (SPIM) setup the development of multiple embryos is recorded in parallel and a map of all labelled cells is obtained for each embryo in <10 s. In these panoramic projections, cell segmentation and flow analysis reveal characteristic migration patterns and global tissue remodelling in the early endoderm. Merging data from many samples uncover stereotypic patterns that are fundamental to endoderm development in every embryo. We demonstrate that processing and compressing raw image data in real time is not only efficient but indispensable for image-based systems biology.
Figures


References
-
- Lecuit T. Tissue Remodeling and Epithelial Morphogenesis. Current Topics in Developmental Biology Academic Press (2009).
-
- Weber M. & Huisken J. Light sheet microscopy for real-time developmental biology. Curr. Opin. Genet. Dev. 21, 566–572 (2011). - PubMed
-
- Huisken J., Swoger J., Del Bene F., Wittbrodt J. & Stelzer E. H. K. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305, 1007–1009 (2004). - PubMed
-
- Swoger J., Verveer P. J., Greger K., Huisken J. & Stelzer E. H. K. Multi-view image fusion improves resolution in three-dimensional microscopy. Opt. Express 15, 8029–8042 (2007). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

