Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013 - United States
- PMID: 23884346
- PMCID: PMC4604972
Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013 - United States
Abstract
Since mid-2006, the Advisory Committee on Immunization Practices (ACIP) has recommended routine vaccination of adolescent girls at ages 11 or 12 years with 3 doses of human papillomavirus (HPV) vaccine. Two HPV vaccines are currently available in the United States. Both the quadrivalent (HPV4) and bivalent (HPV2) vaccines protect against HPV types 16 and 18, which cause 70% of cervical cancers and the majority of other HPV-associated cancers; HPV4 also protects against HPV types 6 and 11, which cause 90% of genital warts.* This report summarizes national HPV vaccination coverage levels among adolescent girls aged 13-17 years† from the 2007-2012 National Immunization Survey-Teen (NIS-Teen) and national postlicensure vaccine safety monitoring. Although vaccination coverage with ≥1 dose of any HPV vaccine increased from 25.1% in 2007 to 53.0% in 2011, coverage in 2012 (53.8%) was similar to 2011. If HPV vaccine had been administered during health-care visits when another vaccine was administered, vaccination coverage for ≥1 dose could have reached 92.6%. Safety monitoring data continue to indicate that HPV4 is safe. Despite availability of safe and effective vaccines and ample opportunities for vaccine delivery in the health-care setting, HPV vaccination coverage among adolescent girls failed to increase from 2011 to 2012.
Figures
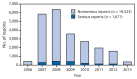
References
-
- CDC. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2007;56(RR-2) - PubMed
-
- Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750–7. - PubMed
-
- CDC. National and state vaccination coverage among adolescents aged 13–17 years—United States, 2011. MMWR. 2012;61:671–7. - PubMed
-
- Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187–93. - PubMed
-
- Markowitz LE, Hariri S, Lin C, et al. Reduction in HPV prevalence among young women following vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208:385–93. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

