Notch/Rbpjκ signaling regulates progenitor maintenance and differentiation of hypothalamic arcuate neurons
- PMID: 23884446
- PMCID: PMC3742139
- DOI: 10.1242/dev.098681
Notch/Rbpjκ signaling regulates progenitor maintenance and differentiation of hypothalamic arcuate neurons
Abstract
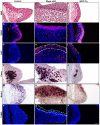
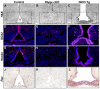
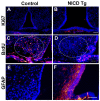
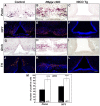
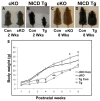
The hypothalamic arcuate nucleus (Arc), containing pro-opoiomelanocortin (POMC), neuropeptide Y (NPY) and growth hormone releasing hormone (GHRH) neurons, regulates feeding, energy balance and body size. Dysregulation of this homeostatic mediator underlies diseases ranging from growth failure to obesity. Despite considerable investigation regarding the function of Arc neurons, mechanisms governing their development remain unclear. Notch signaling factors such as Hes1 and Mash1 are present in hypothalamic progenitors that give rise to Arc neurons. However, how Notch signaling controls these progenitor populations is unknown. To elucidate the role of Notch signaling in Arc development, we analyzed conditional loss-of-function mice lacking a necessary Notch co-factor, Rbpjκ, in Nkx2.1-cre-expressing cells (Rbpjκ cKO), as well as mice with expression of the constitutively active Notch1 intracellular domain (NICD) in Nkx2.1-cre-expressing cells (NICD Tg). We found that loss of Rbpjκ results in absence of Hes1 but not of Hes5 within the primordial Arc at E13.5. Additionally, Mash1 expression is increased, coincident with increased proliferation and accumulation of Arc neurons at E13.5. At E18.5, Rbpjκ cKO mice have few progenitors and show increased numbers of differentiated Pomc, NPY and Ghrh neurons. By contrast, NICD Tg mice have increased hypothalamic progenitors, show an absence of differentiated Arc neurons and aberrant glial differentiation at E18.5. Subsequently, both Rbpjκ cKO and NICD Tg mice have changes in growth and body size during postnatal development. Taken together, our results demonstrate that Notch/Rbpjκ signaling regulates the generation and differentiation of Arc neurons, which contribute to homeostatic regulation of body size.
Keywords: Arcuate; Hypothalamus; Mouse; NPY; Notch; POMC.
Figures



References
-
- Akazawa C., Sasai Y., Nakanishi S., Kageyama R. (1992). Molecular characterization of a rat negative regulator with a basic helix-loop-helix structure predominantly expressed in the developing nervous system. J. Biol. Chem. 267, 21879–21885 - PubMed
-
- Akimoto M., Nishimaki T., Arai Y., Uchinuma E., Yamauchi H., Kameda Y. (2010). Hes1 regulates formations of the hypophyseal pars tuberalis and the hypothalamus. Cell Tissue Res. 340, 509–521 - PubMed
-
- Altman J., Bayer S. A. (1978). Development of the diencephalon in the rat. I. Autoradiographic study of the time of origin and settling patterns of neurons of the hypothalamus. J. Comp. Neurol. 182, 945–971 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

