NCR3/NKp30 contributes to pathogenesis in primary Sjogren's syndrome
- PMID: 23884468
- PMCID: PMC4237161
- DOI: 10.1126/scitranslmed.3005727
NCR3/NKp30 contributes to pathogenesis in primary Sjogren's syndrome
Abstract
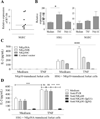
Primary Sjögren's syndrome (pSS) is a chronic autoimmune disease characterized by a lymphocytic exocrinopathy. However, patients often have evidence of systemic autoimmunity, and they are at markedly increased risk for the development of non- Hodgkin's lymphoma. Similar to other autoimmune disorders, a strong interferon (IFN) signature is present among subsets of pSS patients, although the precise etiology remains uncertain. NCR3/NKp30 is a natural killer (NK)-specific activating receptor regulating the cross talk between NK and dendritic cells and type II IFN secretion. We performed a case-control study of genetic polymorphisms of the NCR3/NKp30 gene and found that rs11575837 (G>A) residing in the promoter was associated with reduced gene transcription and function as well as protection to pSS. We also demonstrated that circulating levels of NCR3/NKp30 were significantly increased among pSS patients compared with controls and correlated with higher NCR3/NKp30 but not CD16-dependent IFN-γ secretion by NK cells. Excess accumulation of NK cells in minor salivary glands correlated with the severity of the exocrinopathy. B7H6, the ligand of NKp30, was expressed by salivary epithelial cells. These findings suggest that NK cells may promote an NKp30-dependent inflammatory state in salivary glands and that blockade of the B7H6/NKp30 axis could be clinically relevant in pSS.
Figures





Comment in
-
When killers become helpers.Sci Transl Med. 2013 Jul 24;5(195):195fs29. doi: 10.1126/scitranslmed.3006850. Sci Transl Med. 2013. PMID: 23884464 Free PMC article.
References
-
- Wildenberg ME, Welzen-Coppens JM, van Helden-Meeuwsen CG, Bootsma H, Vissink A, van Rooijen N, van de Merwe JP, Drexhage HA, Versnel MA. Increased frequency of CD16+ monocytes and the presence of activated dendritic cells in salivary glands in primary Sjogren syndrome. Ann Rheum Dis. 2009 Mar;68:420. - PubMed
-
- Dawson LJ, Fox PC, Smith PM. Sjogrens syndrome--the non-apoptotic model of glandular hypofunction. Rheumatology (Oxford) 2006 Jul;45:792. - PubMed
-
- Delaleu N, Jonsson MV, Appel S, Jonsson R. New concepts in the pathogenesis of Sjogren’s syndrome. Rheum Dis Clin North Am. 2008 Nov;34:833. - PubMed
-
- Bave U, Nordmark G, Lovgren T, Ronnelid J, Cajander S, Eloranta ML, Alm GV, Ronnblom L. Activation of the type I interferon system in primary Sjogren’s syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 2005 Apr;52:1185. - PubMed
-
- Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, Jacques S, Ba N, Ittah M, Lepajolec C, Labetoulle M, Ardizzone M, Sibilia J, Fournier C, Chiocchia G, Mariette X. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren’s syndrome. Proc Natl Acad Sci U S A. 2006 Feb 21;103:2770. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DE015223/DE/NIDCR NIH HHS/United States
- P50 AR0608040/AR/NIAMS NIH HHS/United States
- 3P20 RR020143/RR/NCRR NIH HHS/United States
- 5U19 AI082714/AI/NIAID NIH HHS/United States
- P01 AI083194/AI/NIAID NIH HHS/United States
- R01 DE018209/DE/NIDCR NIH HHS/United States
- 5P01 AI083194-03/AI/NIAID NIH HHS/United States
- P20 RR020143/RR/NCRR NIH HHS/United States
- 5R01 DE015223/DE/NIDCR NIH HHS/United States
- U19 AI082714/AI/NIAID NIH HHS/United States
- P50 AR060804/AR/NIAMS NIH HHS/United States
- 5R01 DE018209/DE/NIDCR NIH HHS/United States
- 1R01 DE018209-02/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

