CaMKII is essential for the proasthmatic effects of oxidation
- PMID: 23884469
- PMCID: PMC4331168
- DOI: 10.1126/scitranslmed.3006135
CaMKII is essential for the proasthmatic effects of oxidation
Abstract
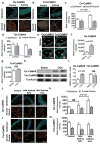
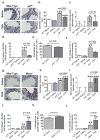
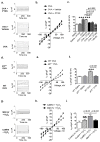
Increased reactive oxygen species (ROS) contribute to asthma, but little is known about the molecular mechanisms connecting increased ROS with characteristic features of asthma. We show that enhanced oxidative activation of the Ca(2+)/calmodulin-dependent protein kinase (ox-CaMKII) in bronchial epithelium positively correlates with asthma severity and that epithelial ox-CaMKII increases in response to inhaled allergens in patients. We used mouse models of allergic airway disease induced by ovalbumin (OVA) or Aspergillus fumigatus (Asp) and found that bronchial epithelial ox-CaMKII was required to increase a ROS- and picrotoxin-sensitive Cl(-) current (ICl) and MUC5AC expression, upstream events in asthma progression. Allergen challenge increased epithelial ROS by activating NADPH oxidases. Mice lacking functional NADPH oxidases due to knockout of p47 and mice with epithelial-targeted transgenic expression of a CaMKII inhibitory peptide or wild-type mice treated with inhaled KN-93, an experimental small-molecule CaMKII antagonist, were protected against increases in ICl, MUC5AC expression, and airway hyperreactivity to inhaled methacholine. Our findings support the view that CaMKII is a ROS-responsive, pluripotent proasthmatic signal and provide proof-of-concept evidence that CaMKII is a therapeutic target in asthma.
Conflict of interest statement
M.E.A., I.M.G., and J.N.K. are named inventors on a patent application from The University of Iowa claiming to treat asthma by CaMKII inhibition. Methods of Treating Pulmonary Diseases and Disorders by Modulating Calcium/Calmodulin Dependent Protein Kinase II Activity, Patent Number US 20110152172 A1. M.E.A. is a cofounder of Allosteros Therapeutics, a biotech aiming to develop enzyme inhibitor therapies. The remaining authors declare no competing interests.
Figures



References
-
- Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127:145–152. - PubMed
-
- Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, Sigmund R, Seibold W, Moroni-Zentgraf P, Bateman ED. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367:1198–1207. - PubMed
-
- Jarjour NN, Calhoun WJ. Enhanced production of oxygen radicals in asthma. J Lab Clin Med. 1994;123:131–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

